
Composites Materials for Food Packaging
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Composites Materials for Food Packaging
About this book
The novel insights, as well as the main drawbacks of each engineered composites material is extensively evaluated taking into account the strong relationship between packaging materials, environmental and reusability concerns, food quality, and nutritional value.
Composites, by matching the properties of different components, allow the development of innovative and performing strategies for intelligent food packaging, thus overcoming the limitations of using only a single material.
The book starts with the description of montmorillonite and halloysite composites, subsequently moving to metal-based materials with special emphasis on silver, zinc, silicium and iron. After the discussion about how the biological influences of such materials can affect the performance of packaging, the investigation of superior properties of sp 2 carbon nanostructures is reported. Here, carbon nanotubes and graphene are described as starting points for the preparation of highly engineered composites able to promote the enhancement of shelf-life by virtue of their mechanical and electrical features.
Finally, in the effort to find innovative composites, the applicability of biodegradable materials from both natural (e.g. cellulose) and synthetic (e.g. polylactic acid – PLA) origins, with the aim to prove that polymer-based materials can overcome some key limitations such as environmental impact and waste disposal.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
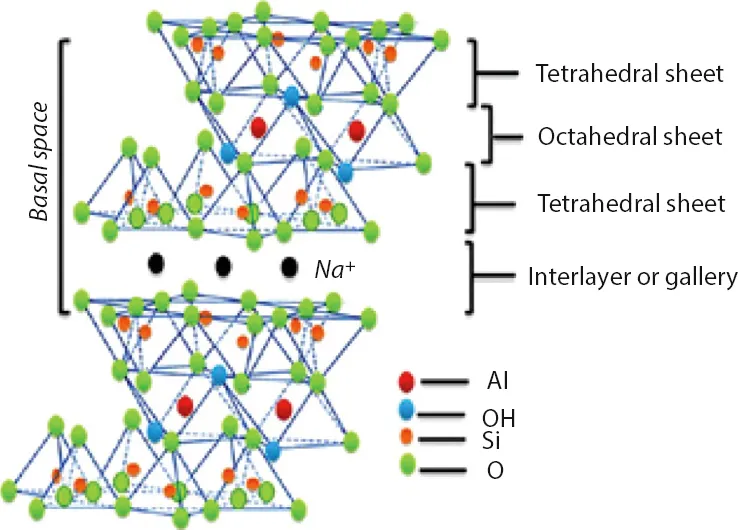
Montmorillonite Composite Materials and Food Packaging
Abstract
1.1 Introduction
| Name | Chemical composition – Basic chemical formula | Modifier and modifier concentration |
| Cloisites Na+ | Montmorillonite (MMT) Na0.2Ca0.1Al2Si4O10(OH)2(H2O)10 | None |
| Cloisites 30B | Modified MMT | Quaternary ammonium salt (MT2EtOH) 90 meq/100 g clay |
| Cloisites 20A | Modified MMT | Quaternary ammonium salt (2M2HT) 95 meq/100 g clay |
| Cloisites 93A | Modified MMT | Ternary ammonium salt (M2HT) 90 meq/100 g clay |
| Cloisites 15A | Modified MMT | Quaternary ammonium salt (2M2HT) 125 meq/100 g clay |
| Cloisites 10A | Modified MMT | Quaternary ammonium salt (2MBHT) 125 meq/100 g clay |
| Clay1 | Modified MMT | Quaternary ammonium salt (HDTA)6-fold the CEC of raw clay (raw clay with a cation exchange capacity (CEC)¼ 92.6 meq/100 g clay) |
| Clay2 | Modified MMT | Quaternary ammonium salt (HDTAϸACO)HDTA in 5.75-fold and ACO in 0.25-fold of the CEC of raw clay (92.6 meq/100 g clay) |
| Oligo(styrene-co-acrylonitrile) MMT | Modified MMT | Quaternary ammonium salt of poly(styrene-co-acrylonitrile)(CEC¼ 0.9 meg/g clay) |
Table of contents
- Cover
- Title page
- Copyright page
- Preface
- Chapter 1: Montmorillonite Composite Materials and Food Packaging
- Chapter 2: Halloysite Containing Composites for Food Packaging Applications
- Chapter 3: Silver Composite Materials and Food Packaging
- Chapter 4: Zinc Composite Materials and Food Packaging
- Chapter 5: Silicium-Based Nanocomposite Materials for Food Packaging Applications
- Chapter 6: Nanoiron-Based Composite Oxygen Scavengers for Food Packaging
- Chapter 7: Carbon Nanotubes (CNTs) Composite Materials and Food Packaging
- Chapter 8: Polymer/Graphene Nanocomposites for Food Packaging
- Chapter 9: Biodegradability and Compostability of Food Nanopackaging Materials
- Chapter 10: Nanocellulose in Food Packaging
- Chapter 11: Nanocellulose in Combination with Inorganic/Organic Biocides for Food Film Packaging Applications – Safety Issues Review
- Chapter 12: Composite Materials Based on PLA and its Applications in Food Packaging
- Chapter 13: Nanomaterial Migration from Composites into Food Matrices
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app