
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemical Fundamentals of Geology and Environmental Geoscience
About this book
Chemical principles are fundamental to the Earth sciences, and geoscience students increasingly require a firm grasp of basic chemistry to succeed in their studies. The enlarged third edition of this highly regarded textbook introduces the student to such 'geo-relevant' chemistry, presented in the same lucid and accessible style as earlier editions, but the new edition has been strengthened in its coverage of environmental geoscience and incorporates a new chapter introducing isotope geochemistry.
The book comprises three broad sections. The first (Chapters 1–4) deals with the basic physical chemistry of geological processes. The second (Chapters 5–8) introduces the wave-mechanical view of the atom and explains the various types of chemical bonding that give Earth materials their diverse and distinctive properties. The final chapters (9–11) survey the geologically relevant elements and isotopes, and explain their formation and their abundances in the cosmos and the Earth. The book concludes with an extensive glossary of terms; appendices cover basic maths, explain basic solution chemistry, and list the chemical elements and the symbols, units and constants used in the book.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
ENERGY IN GEOCHEMICAL PROCESSES
Introduction
Box 1.1 What is energy?
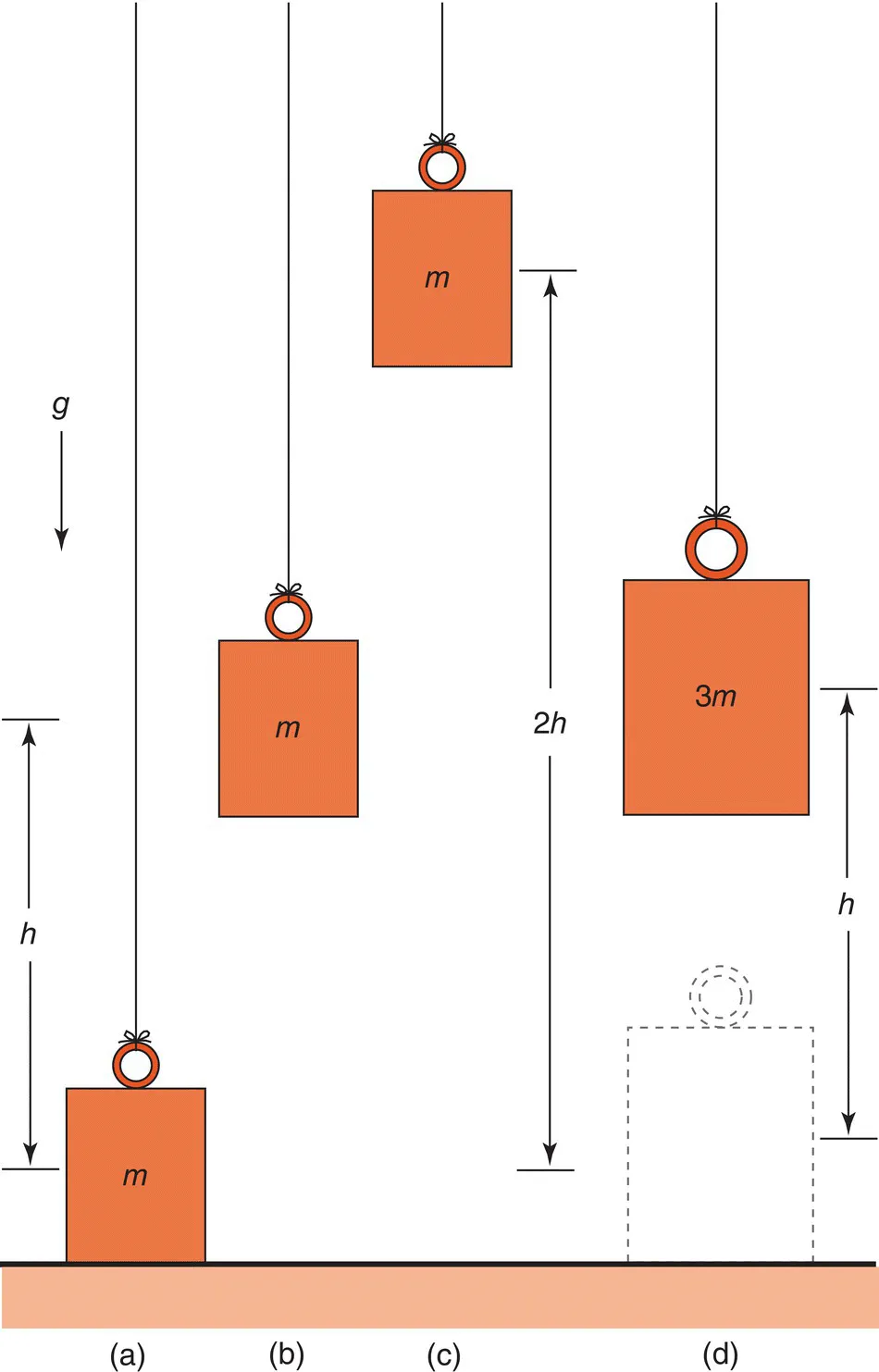
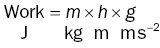
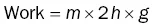
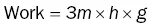
Table of contents
- COVER
- TITLE PAGE
- COPYRIGHT PAGE
- PREFACE TO THE THIRD EDITION
- PREFACE TO THE SECOND EDITION
- PREFACE TO THE FIRST EDITION
- ACKNOWLEDGEMENTS
- 1 ENERGY IN GEOCHEMICAL PROCESSES
- 2 EQUILIBRIUM IN GEOLOGICAL SYSTEMS
- 3 KINETICS OF EARTH PROCESSES
- 4 AQUEOUS SOLUTIONS AND THE HYDROSPHERE
- 5 ELECTRONS IN ATOMS
- 6 WHAT WE CAN LEARN FROMTHE PERIODIC TABLE
- 7 CHEMICAL BONDING AND THE PROPERTIES OF MINERALS
- 8 SILICATE CRYSTALS AND MELTS
- 9 SOME GEOLOGICALLY IMPORTANT ELEMENTS
- 10 WHAT CAN WE LEARN FROM ISOTOPES?
- 11 THE ELEMENTS IN THE UNIVERSE
- ANSWERS TO EXERCISES
- APPENDIX A: MATHEMATICS REVISION
- APPENDIX B: SIMPLE SOLUTION CHEMISTRY
- APPENDIX C: ALPHABETICAL LIST OF CHEMICAL ABBREVIATIONS AND ELEMENT NAMES, WITH ATOMIC NUMBER AND RELATIVE ATOMIC MASS
- APPENDIX D: SYMBOLS, UNITS, CONSTANTS AND ABBREVIATIONS USED IN THIS BOOK
- GLOSSARY
- REFERENCES
- SUPPLEMENTAL IMAGES
- INDEX
- ACCESS TO COMPANION SITE
- END USER LICENSE AGREEMENT