
eBook - ePub
Antibiotic Drug Resistance
José-Luis Capelo-Martínez, Gilberto Igrejas, José-Luis Capelo-Martínez, Gilberto Igrejas
This is a test
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Antibiotic Drug Resistance
José-Luis Capelo-Martínez, Gilberto Igrejas, José-Luis Capelo-Martínez, Gilberto Igrejas
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
This book presents a thorough and authoritative overview of the multifaceted field of antibiotic science – offering guidance to translate research into tools for prevention, diagnosis, and treatment of infectious diseases.
- Provides readers with knowledge about the broad field of drug resistance
- Offers guidance to translate research into tools for prevention, diagnosis, and treatment of infectious diseases
- Links strategies to analyze microbes to the development of new drugs, socioeconomic impacts to therapeutic strategies, and public policies to antibiotic-resistance-prevention strategies
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Antibiotic Drug Resistance als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Antibiotic Drug Resistance von José-Luis Capelo-Martínez, Gilberto Igrejas, José-Luis Capelo-Martínez, Gilberto Igrejas im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Medicina & Farmacología. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Part I
Current Antibiotics and Their Mechanism of Action
1
Resistance to Aminoglycosides: Glycomics and the Link to the Human Gut Microbiome
Viviana G. Correia Benedita A. Pinheiro Ana Luísa Carvalho, and Angelina S. Palma
UCIBIO‐REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade NOVA de Lisboa, Caparica, Portugal
1.1 Aminoglycosides as Antimicrobial Drugs
The exponential appearance of antibiotic‐resistant infections, in particular those caused by Gram‐negative pathogens, is a major public health concern. The observed decrease in the emergence of new effective antimicrobial drugs is an inevitable consequence of the use of antibiotics, and new approaches to fight infection are a matter in need of attention from the scientific community (Magiorakos et al. 2012). In response to this challenge, the optimization of existing drugs with known mechanisms of action and resistance, such as aminoglycosides, is an attractive approach for the development of new antimicrobials.
Aminoglycosides or aminoglycoside antibiotics (AGAs) are secondary metabolites of bacteria used in the warfare against other microorganisms, which were repurposed in medicine as broad‐spectrum antibiotics in both humans and animals. This class of antibiotics has activity against Gram‐negative and Gram‐positive bacteria by targeting ribosomal RNA (rRNA), leading to protein misfolding. AGAs have predictable pharmacokinetics and often act in synergy with other antibiotics, such as beta‐lactams, making them powerful anti‐infective drugs (Hanberger et al. 2013). Despite their potential renal toxicity and ototoxicity and known bacterial resistance, diverse molecules of this family of antibiotics have been used in clinical practice for several decades (Thamban Chandrika and Garneau‐Tsodikova 2018).
AGAs are constituted by a carbohydrate residue moiety and possess several amino and hydroxyl group functionalities, determinants for the interaction with target sequences on the rRNA and for impairing normal ribosomal function. Although natural AGAs share the same myo‐inositol‐based core (Figure 1.1), these molecules exhibit significant structural differences depending on the bacterial origin, which result in different biological activities. Importantly, the bacterial origin is also the driving force behind bacterial resistance, as it enables bacteria to alter the structure of AGAs by modifying their amino and hydroxyl groups.
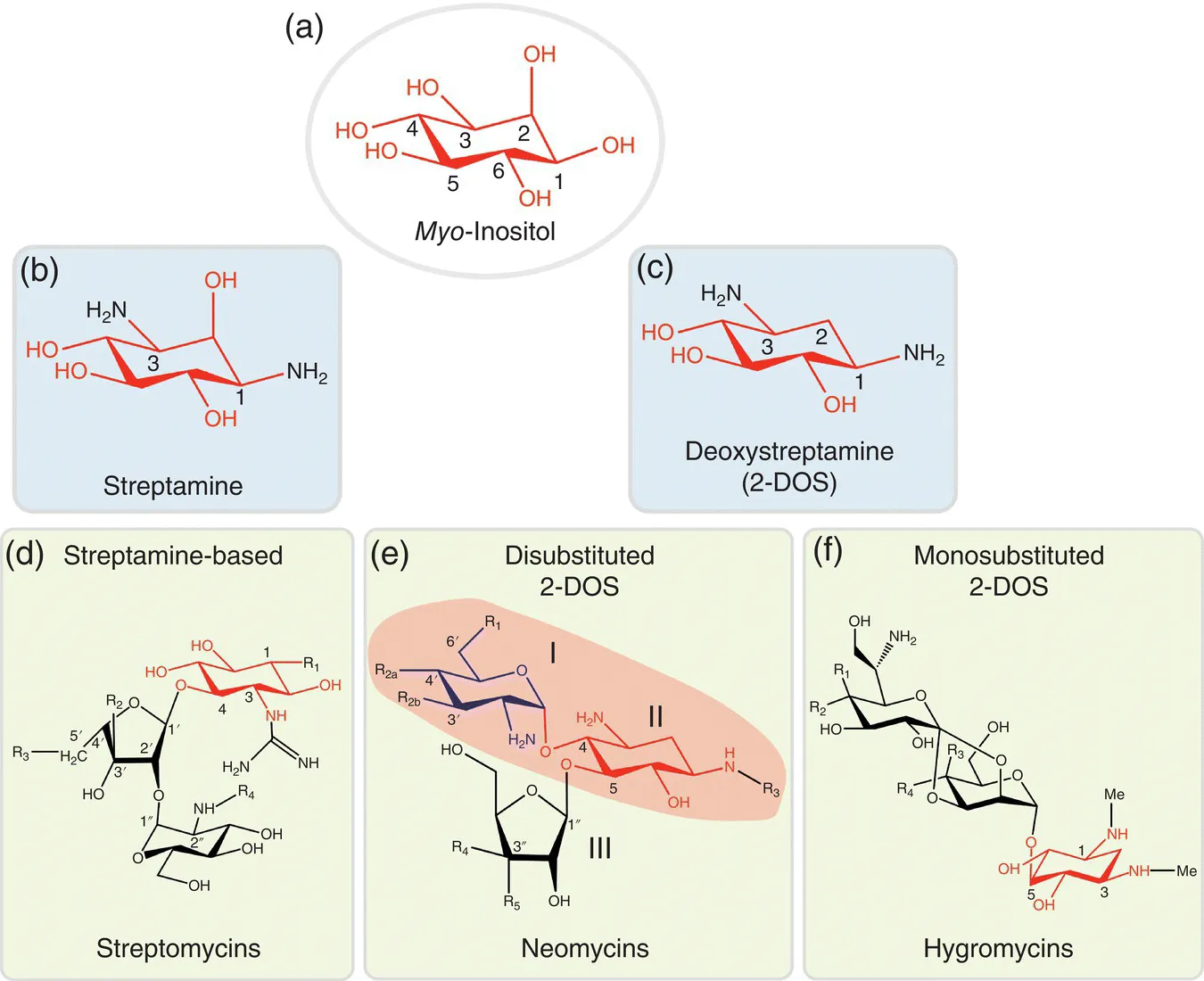
Figure 1.1 Core structural elements of the aminoglycosides and examples of clinically relevant AGA families. Each family has a primary structure with different substitutions (Rn) at hydroxyl and amino groups. The rings (I–III) of the neomycins' representative structure are numbered as usually observed in the literature for disubstituted 2‐DOS AGAs. In panel (e), the pseudodisaccharide core structure paromamine, also termed as neamine, is demarked.
Streptomycin was the first identified and characterized AGA and the first useful antibiotic obtained from a bacterial source (1944). This AGA was isolated from the soil‐dwelling bacterial species Streptomyces and Micromonospora and successfully introduced into clinical practice in 1940 to treat tuberculosis. After the initial discovery of streptomycin and its streptamine‐based relatives (Figure 1.1a), several others followed, and the development of bacterial resistance was largely overcome by introduction of AGAs derived from 2‐deoxystreptamine (DOS) (Figure 1.1c), reviewed in (Davies 2007). These included neomycin (1949), kanamycin (1957), gentamycin (1963), tobramycin (1967), and sisomicin (1970). The acquisition of bacterial resistance for the DOS aminoglycosides prompted the development of novel and potent semisynthetic AGAs. These second‐generation AGAs resulted from the insertion of a 4‐hydroxy‐2‐aminobutyric acid (HABA) substituent of the C‐1 amine group on the DOS ring of kanamycin and gentamycin‐derived compounds. Examples are dibekacin (1971), amikacin (1972), arbekacin (1973), isepamicin (1975), and netilmicin (1976). However, because of their clinical usage, bacteria also developed resistance mechanisms against these semisynthetic antibiotics, almost leading to the abandon of AGAs.
Recently, the interest in AGAs research has resurged as consequence of the increasing number of strains resistant to other classes of antibiotics, such as the Gram‐negative bacteria Enterococcus faecium responsible for serious invasive nosocomial infections (Buelow et al. 2017). New approaches have been used for developing semisynthetic AGAs using combined structure–activity relationship (SAR), in search for less toxic but effective AGAs (Thamban Chandrika and Garneau‐Tsodikova 2018). The AGA plazomicin developed by Achaogen Inc. (ACHN‐490) (Aggen et al. 2010), currently in phase III clinical trials, is an evidence of the renewed interest. Table 1.1 summarizes described AGAs and their distinctive features.
Table 1.1 Overview of major aminoglycoside antibiotics (AGAs) and their distinctive features and effect on the human gut microbiome.
Source: Adapted from Piepersberg et al. (2007) and Becker and Cooper (2013).
| AGA | Core‐derived structure | Common use | Effect on human gut microbiome | Related pathology or disease | Microbiome‐related studies |
| Naturally occurring | |||||
| Apramycin (APR) | 4‐Monosubstituted 2‐DOS | Veterinary | NA | NA | NA |
| Butirosin (BTR) | 4,5‐Disubstituted 2‐DOS | Biochemical reagent | NA | NA | NA |
| Fortimicin (... | |||||
Inhaltsverzeichnis
Zitierstile für Antibiotic Drug Resistance
APA 6 Citation
[author missing]. (2019). Antibiotic Drug Resistance (1st ed.). Wiley. Retrieved from https://www.perlego.com/book/1148821/antibiotic-drug-resistance-pdf (Original work published 2019)
Chicago Citation
[author missing]. (2019) 2019. Antibiotic Drug Resistance. 1st ed. Wiley. https://www.perlego.com/book/1148821/antibiotic-drug-resistance-pdf.
Harvard Citation
[author missing] (2019) Antibiotic Drug Resistance. 1st edn. Wiley. Available at: https://www.perlego.com/book/1148821/antibiotic-drug-resistance-pdf (Accessed: 14 October 2022).
MLA 7 Citation
[author missing]. Antibiotic Drug Resistance. 1st ed. Wiley, 2019. Web. 14 Oct. 2022.