
eBook - ePub
Antibiotic Drug Resistance
José-Luis Capelo-Martínez, Gilberto Igrejas, José-Luis Capelo-Martínez, Gilberto Igrejas
This is a test
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Antibiotic Drug Resistance
José-Luis Capelo-Martínez, Gilberto Igrejas, José-Luis Capelo-Martínez, Gilberto Igrejas
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
This book presents a thorough and authoritative overview of the multifaceted field of antibiotic science – offering guidance to translate research into tools for prevention, diagnosis, and treatment of infectious diseases.
- Provides readers with knowledge about the broad field of drug resistance
- Offers guidance to translate research into tools for prevention, diagnosis, and treatment of infectious diseases
- Links strategies to analyze microbes to the development of new drugs, socioeconomic impacts to therapeutic strategies, and public policies to antibiotic-resistance-prevention strategies
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Antibiotic Drug Resistance est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Antibiotic Drug Resistance par José-Luis Capelo-Martínez, Gilberto Igrejas, José-Luis Capelo-Martínez, Gilberto Igrejas en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Medicina et Farmacología. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Part I
Current Antibiotics and Their Mechanism of Action
1
Resistance to Aminoglycosides: Glycomics and the Link to the Human Gut Microbiome
Viviana G. Correia Benedita A. Pinheiro Ana Luísa Carvalho, and Angelina S. Palma
UCIBIO‐REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade NOVA de Lisboa, Caparica, Portugal
1.1 Aminoglycosides as Antimicrobial Drugs
The exponential appearance of antibiotic‐resistant infections, in particular those caused by Gram‐negative pathogens, is a major public health concern. The observed decrease in the emergence of new effective antimicrobial drugs is an inevitable consequence of the use of antibiotics, and new approaches to fight infection are a matter in need of attention from the scientific community (Magiorakos et al. 2012). In response to this challenge, the optimization of existing drugs with known mechanisms of action and resistance, such as aminoglycosides, is an attractive approach for the development of new antimicrobials.
Aminoglycosides or aminoglycoside antibiotics (AGAs) are secondary metabolites of bacteria used in the warfare against other microorganisms, which were repurposed in medicine as broad‐spectrum antibiotics in both humans and animals. This class of antibiotics has activity against Gram‐negative and Gram‐positive bacteria by targeting ribosomal RNA (rRNA), leading to protein misfolding. AGAs have predictable pharmacokinetics and often act in synergy with other antibiotics, such as beta‐lactams, making them powerful anti‐infective drugs (Hanberger et al. 2013). Despite their potential renal toxicity and ototoxicity and known bacterial resistance, diverse molecules of this family of antibiotics have been used in clinical practice for several decades (Thamban Chandrika and Garneau‐Tsodikova 2018).
AGAs are constituted by a carbohydrate residue moiety and possess several amino and hydroxyl group functionalities, determinants for the interaction with target sequences on the rRNA and for impairing normal ribosomal function. Although natural AGAs share the same myo‐inositol‐based core (Figure 1.1), these molecules exhibit significant structural differences depending on the bacterial origin, which result in different biological activities. Importantly, the bacterial origin is also the driving force behind bacterial resistance, as it enables bacteria to alter the structure of AGAs by modifying their amino and hydroxyl groups.
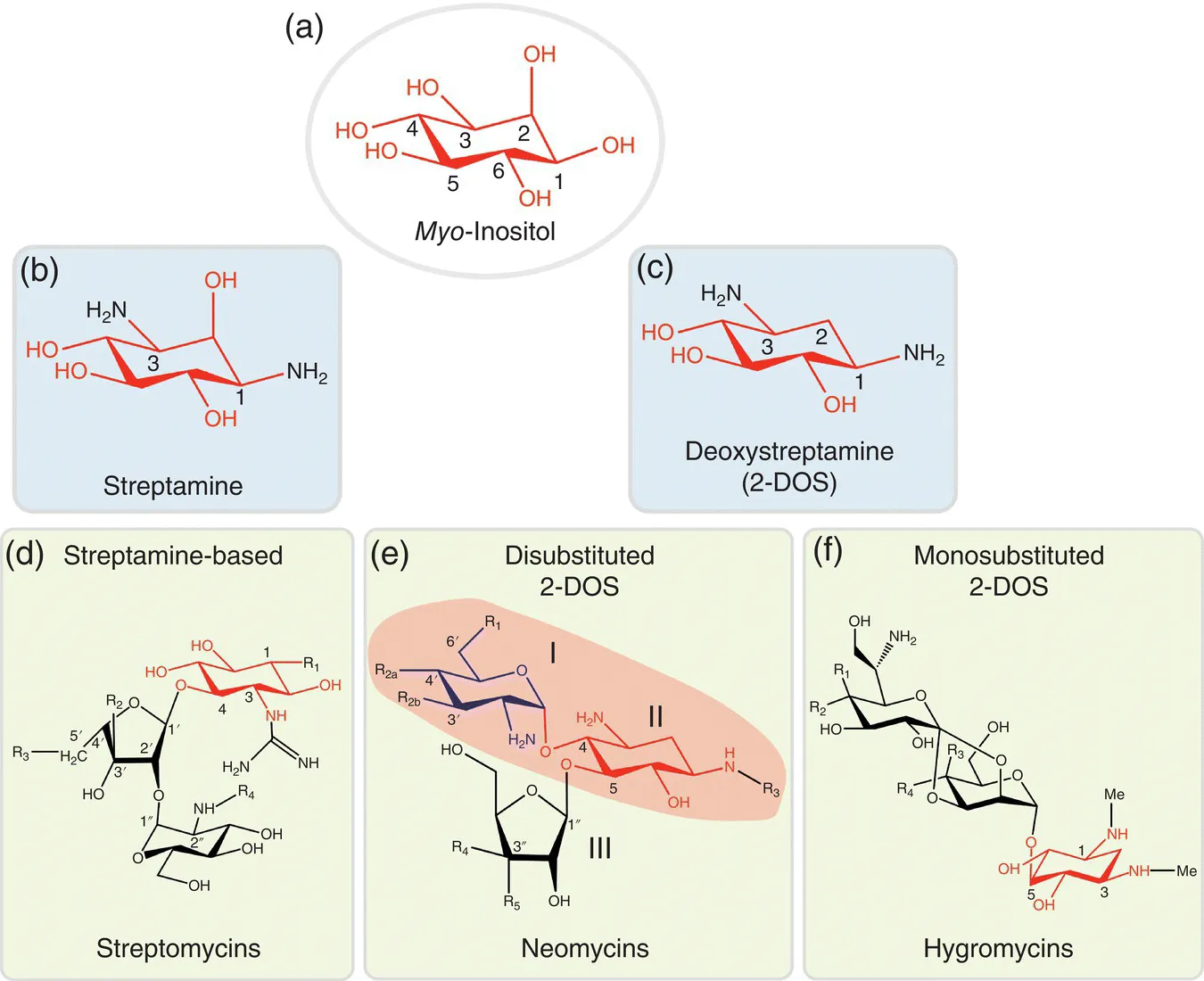
Figure 1.1 Core structural elements of the aminoglycosides and examples of clinically relevant AGA families. Each family has a primary structure with different substitutions (Rn) at hydroxyl and amino groups. The rings (I–III) of the neomycins' representative structure are numbered as usually observed in the literature for disubstituted 2‐DOS AGAs. In panel (e), the pseudodisaccharide core structure paromamine, also termed as neamine, is demarked.
Streptomycin was the first identified and characterized AGA and the first useful antibiotic obtained from a bacterial source (1944). This AGA was isolated from the soil‐dwelling bacterial species Streptomyces and Micromonospora and successfully introduced into clinical practice in 1940 to treat tuberculosis. After the initial discovery of streptomycin and its streptamine‐based relatives (Figure 1.1a), several others followed, and the development of bacterial resistance was largely overcome by introduction of AGAs derived from 2‐deoxystreptamine (DOS) (Figure 1.1c), reviewed in (Davies 2007). These included neomycin (1949), kanamycin (1957), gentamycin (1963), tobramycin (1967), and sisomicin (1970). The acquisition of bacterial resistance for the DOS aminoglycosides prompted the development of novel and potent semisynthetic AGAs. These second‐generation AGAs resulted from the insertion of a 4‐hydroxy‐2‐aminobutyric acid (HABA) substituent of the C‐1 amine group on the DOS ring of kanamycin and gentamycin‐derived compounds. Examples are dibekacin (1971), amikacin (1972), arbekacin (1973), isepamicin (1975), and netilmicin (1976). However, because of their clinical usage, bacteria also developed resistance mechanisms against these semisynthetic antibiotics, almost leading to the abandon of AGAs.
Recently, the interest in AGAs research has resurged as consequence of the increasing number of strains resistant to other classes of antibiotics, such as the Gram‐negative bacteria Enterococcus faecium responsible for serious invasive nosocomial infections (Buelow et al. 2017). New approaches have been used for developing semisynthetic AGAs using combined structure–activity relationship (SAR), in search for less toxic but effective AGAs (Thamban Chandrika and Garneau‐Tsodikova 2018). The AGA plazomicin developed by Achaogen Inc. (ACHN‐490) (Aggen et al. 2010), currently in phase III clinical trials, is an evidence of the renewed interest. Table 1.1 summarizes described AGAs and their distinctive features.
Table 1.1 Overview of major aminoglycoside antibiotics (AGAs) and their distinctive features and effect on the human gut microbiome.
Source: Adapted from Piepersberg et al. (2007) and Becker and Cooper (2013).
| AGA | Core‐derived structure | Common use | Effect on human gut microbiome | Related pathology or disease | Microbiome‐related studies |
| Naturally occurring | |||||
| Apramycin (APR) | 4‐Monosubstituted 2‐DOS | Veterinary | NA | NA | NA |
| Butirosin (BTR) | 4,5‐Disubstituted 2‐DOS | Biochemical reagent | NA | NA | NA |
| Fortimicin (... | |||||