Chapter 1
A Review of the Essentials of Corrosion Needed to Assess Hydrostatic Testing
1.1 “Parallel” and “Series” Corrosion Scenarios
As we can all remember from our school days, and especially from the study of electricity, current can flow in one of two manners depending on whether resistors have been placed one after the other or in parallel with one another. The former, called a “series arrangement,” will show an overall resistance that is the sum of all the resistances, whereas in the latter, the “parallel arrangement,” the overall reciprocal of the resistance is equal to the sum of the reciprocals of the individual resistances.
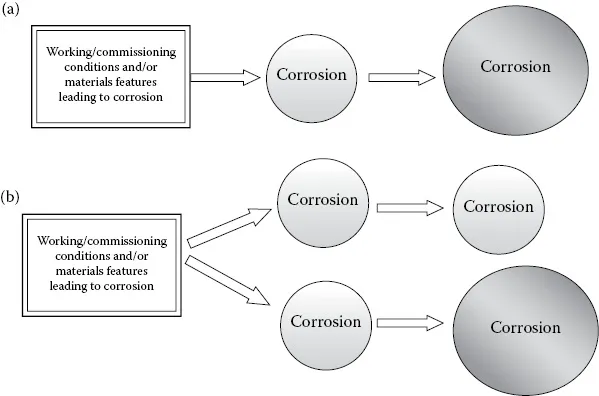
In dealing with corrosion processes we can use the same analogy. Sometimes, corrosive processes can take place electrochemically in a series arrangement, where each will enhance the impact of the other—a synergistic impact. On the other hand, the corrosive processes can also act in a parallel manner, seemingly independently of one another, as shown schematically in Figure 1.1.
Figure 1.1 Corrosion processes arranged in series (a) and in parallel (b). The relative sizes reflect the severity of corrosion.
Figure 1.1 shows that a series corrosion process may simply originate from a corrosion problem that started because of the initial conditions (the chemistry of the system, materials selection, hydrostatic testing [HYD], and so on). This corrosion problem may be aggravated and cause more severe corrosion problems. In a parallel corrosion scenario, however, corrosion processes can evolve independently of each other, and the severity of corrosion (measured in terms of the corrosion rate) may remain the same (upper branch) or become worse (lower branch).
Understanding the concepts of series and parallel corrosion is of vital importance in analyzing the corrosion processes that can become involved during or after HYD is completed.
1.2 Corrosion Scenarios Likely to Develop as a Result of Hydrostatic Testing (HYD) Poor Practice
Corrosion is a “thermodynamically favorable” process, which simply means that the process will happen no matter what the conditions or how perfect the prevention/control measures. Corrosion will happen anyway, and all we can do is control it to make it as slow as possible. This means that the corrosion rates must be so low that, practically speaking, corrosion can be said to have been “under control.”
Anything that assists the “thermodynamically favorable” process of corrosion is undesirable. Factors such as poor welding (in welded parts), the use of maltreated water, inadequate materials selection, and so on could enhance the likelihood of corrosion occurring.
At present, we can address four corrosion scenarios most likely associated with poor HYD (of which microbial corrosion is the most probable) [2]:
▪Microbial corrosion (MIC)
▪Formation of electrochemical cells (oxygen concentration counts)
▪Galvanic corrosion
▪Underdeposit corrosion
We will describe MIC in Chapter 2, but here we will lightly touch on three points that may seem obvious, but are, in fact, quite confusing.
▪Oxygen is an integral part of any electrochemical corrosion. For this reason, oxygen scavengers are added when necessary (see Chapter 3) to exclude oxygen and thus control corrosion.
▪“Dissimilar metals corrosion” is an incorrect terminology and instead of that, “Galvanic corrosion” must be preferred to be used. Galvanic corrosion can occur on the very same metal if conditions allow. A typical example is when a segment of an old pipe is replaced with a new pipe made of the same material. If the pipes have not been taken care of adequately, the new pipe can become the anode and will corrode, despite being the same material as the rest of the pipe.
▪It is possible that the galvanic cell formed as a result of an underdeposit corrosion mechanism such as formation of underbiofilm differential aeration cells will form the required driving force for corrosion. This example alone should serve to show the complexity that may be involved in multiple corrosion reactions, setting some to each other as series and some as parallels. Another example of such is given below.
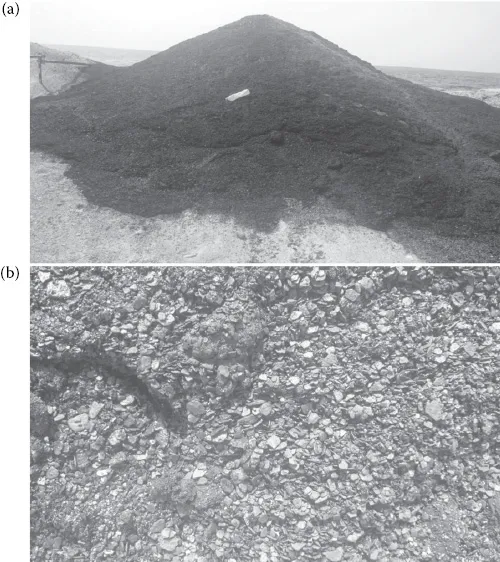
As shown in Figure 1.1, the four corrosion reactions described above can take place in series or in parallel. If, through poor corrosion management, too much deposit is formed inside a pipe, then underdeposit corrosion is inevitable. If, in addition to that, the water used for HYD is maltreated, then by increasing the likelihood of MIC we can face an unfortunate combination of both microbial and non-microbial corrosion taking place inside the pipe. Figure 1.2 shows a situation where the deposits within a pipeline have accumulated due to poor corrosion management and have formed a suitable location at which corrosion can easily proceed.
Figure 1.2 Deposits removed after pigging through a 300 km pipe (a) and a close-up of the overall texture of the deposits (b). (Courtesy of Reza Javaherdashti.)
When welding is done poorly, poor welding and post-welding treatment (PWT) will lead to the formation of platforms upon which corrosion can initiate (with or without HYD). If the conditions are made worse by applying poor HYD using maltreated water for a long period of time, the corrosion that may have happened as a result of the poor welding will be enhanced by an unfortunate combination with microbial corrosion. Figure 1.3 shows welding defects formed during HYD.
Figure 1.3 Example of a failed line pipe manufactured by low-frequency electric resistance welding (LF-ERW) after HYD. (From J.P. Sinha, C.P. Varghese, Assessment of seam integrity of an aging petroleum pipeline constructed with low frequency ERW line pipes, 6th Pipeline Technology Conference 2011, Germany, 2011, http://www.pipeline-conference.com/abstracts/assessment-seam-integrity-aging-petroleum-pipeline-constructed-low-frequency-erw-line. With permission.)
Figure 1.4 presents yet another example of how poor welding practice can result in a post-HYD failure.
Figure 1.4 Brittle fracture during a pipeline hydrotest due to a discontinuity in a submerged arc welding (SAW) longitudinal weld. (From Massimo Benedetto. With permission.)
Therefore, it is essential to realize that what we do can enhance an already existing corrosion problem or will assist in enhancing its detrimental effect on the asset and equipment.
The outcome ...