
eBook - ePub
ABC Proteins
From Bacteria to Man
I Barry Holland, Susan P. C. Cole, Karl Kuchler, Christopher F. Higgins, I Barry Holland, Susan P. C. Cole, Karl Kuchler, Christopher F. Higgins
This is a test
Buch teilen
- 530 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
ABC Proteins
From Bacteria to Man
I Barry Holland, Susan P. C. Cole, Karl Kuchler, Christopher F. Higgins, I Barry Holland, Susan P. C. Cole, Karl Kuchler, Christopher F. Higgins
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
ABC Proteins is an in-depth, up-to-date analysis of all that is known about the subject to date. It discusses and compares evolution, biology and mechanism of action of all known ABC proteins, including the first structural studies as well as clinical implications. It will be useful to anyone trying to stay abreast of the latest findings. This book is sure to become a classic and will regularly be updated.
- Phylogeny and Evoloution of ABC Transporters
- Fundamental Aspects of the Mechanism of Action of ABC Transporters
- Prokaryote ABC Transporters
- Non-Mammalian Transporters
- Multidrug Transporters
- ABC Transporters, Physiological Roles and Human Disease
- Full color throughout
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist ABC Proteins als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu ABC Proteins von I Barry Holland, Susan P. C. Cole, Karl Kuchler, Christopher F. Higgins, I Barry Holland, Susan P. C. Cole, Karl Kuchler, Christopher F. Higgins im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Biological Sciences & Molecular Biology. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Thema
Biological SciencesThema
Molecular BiologyPART I
PHYLOGENY AND EVOLUTION OF ABC TRANSPORTERS
CHAPTER 1
PHYLOGENETIC AND FUNCTIONAL CLASSIFICATION OF ABC (ATP-BINDING CASSETTE) SYSTEMS*
ELIE DASSA
This paper is dedicated to the memory of Maurice Hofnung (1942–2001), a pioneer in the study of ABC (ATP-binding cassette) systems. Two decades ago, by noticing a strong sequence similarity between HisP and MalK, the two first-described ABC proteins, he initiated the studies that led to the identification and characterization of this large superfamily.
INTRODUCTION
ATP-binding cassette (ABC) systems constitute one of the most abundant families of proteins. At the time of writing this review, we have identified more than 2000 ABC ATPase domains or proteins in translated nucleic acid sequence databases. A total of about 6000 proteins were found when the partners of ATPases were taken into account. The size of this mass of sequences is therefore similar to the coding capacity of a bacterial genome. Several properties of members of this superfamily have been reviewed in the last decade (Ames and Lecar, 1992; Ames et al., 1990, 1992; Doige and Ames, 1993; Higgins, 1992; Higgins et al., 1988; Holland and Blight, 1999). The most prominent characteristic of these systems is that they share a highly conserved ATPase domain, the ABC, which has been demonstrated to bind and hydrolyze ATP, thereby providing energy for a large number of biological processes. The amino acid sequence of this cassette displays three major conserved motifs, the Walker A and Walker B motifs commonly found in ATPases together with a specific signature motif, usually commencing LSGG-, and also known as the linker peptide (Schneider and Hunke, 1998). The crystal structures of some ABC proteins are presented in Chapters 4 and 7.
ABC systems are involved not only in the import or export of a wide variety of substances, but also in many cellular processes and in their regulation. Importers constitute mainly the prokaryotic transporters dependent upon a substrate-binding protein (BPD), whose function is to provide bacteria with essential nutrients even if the latter are present in submicromolar concentrations in the environment (Boos and Lucht, 1996). Exporters are found in both prokaryotes and eukaryotes and are involved in the extrusion of noxious substances, the secretion of extracellular toxins and the targeting of membrane components (Fath and Kolter, 1993). The third type of ABC system is apparently not involved in transport but rather in cellular processes such as DNA repair, translation or regulation of gene expression. Since ATP is found principally in the cytosol, we define import as the inwardly directed transport of a molecule into the cytosol. By contrast, export is the translocation of a molecule out of the cytosol, even if its final location is an intracellular organelle. ABC systems of the three types can be distinguished on the basis of the design of their component parts. All the transporters are composed of four structural domains: two very hydrophobic membrane-spanning or integral membrane domains (IMs) and two hydrophilic cytoplasmic domains containing the ABC, peripherally associated with IM on the cytosolic side of the membrane. (a) Importers have in general the four domains encoded as independent polypeptides and they need for function an extracellular substrate-binding protein. (b) In most well-characterized exporters, the transmembrane domains are fused to the ABC domains in several ways. However, some systems with separated IM and ABC domains have been reported to act as exporters although the complete characterization of their transport mechanism awaits more studies. Prokaryote exporters also require accessory proteins and these will be discussed in the specific sections dealing with these transporters. (c) Systems involved in cellular processes other than transport do not have IM domains and are composed of two ABC domains fused together.
INVENTORY AND CLASSIFICATION OF ABC SYSTEMS
To understand the complexity and diversity of ABC systems, computer-assisted methods have been applied by several authors based on comparisons of the ABC ATPase domain, the most highly conserved element. These methods were instrumental in the early definition of the superfamily on the basis of primary sequence comparisons (Higgins et al., 1986). However, in most cases, the ABC proteins of a given organism (Braibant et al., 2000; Linton and Higgins, 1998; Quentin et al., 1999) or ABC systems with clear functional similarity (Fath and Kolter, 1993; Hughes, 1994; Kuan et al., 1995) were compared. The presence of the highly conserved ATPase domain permitted more global comparisons, for example (Paulsen et al., 1998). The first general phylogenetic study specifically devoted to the ABC superfamily (Saurin et al., 1999) was recently updated to include the analysis of about 600 ATPase proteins or domains (Dassa and Bouige, 2001). The sequences segregate in 33 clusters on the phylogenetic tree shown in Figure 1.1. Some clusters comprise obviously highly related proteins known to function together; for example, the two ATPases of oligopeptide importers were fused into a single family. The final 29 families are listed in Table 1.1. Since a general nomenclature for ABC systems is not yet available, Table 1.1 provides the present nomenclature and the equivalent alternative adopted for transporters in general (Saier, 2000) or specifically for human ABC systems (see Chapter 3).
TABLE 1.1
CLASSES, FAMILIES AND SUBFAMILIES OF ABC SYSTEMS
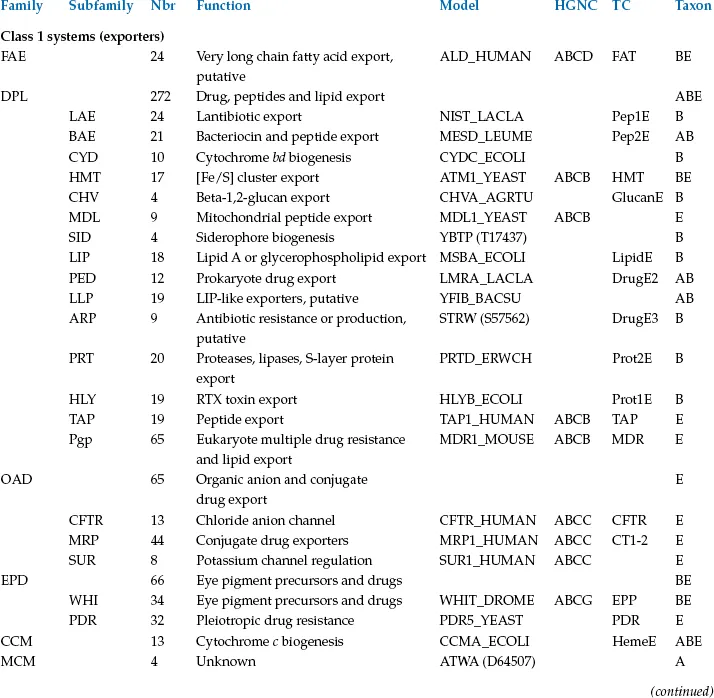
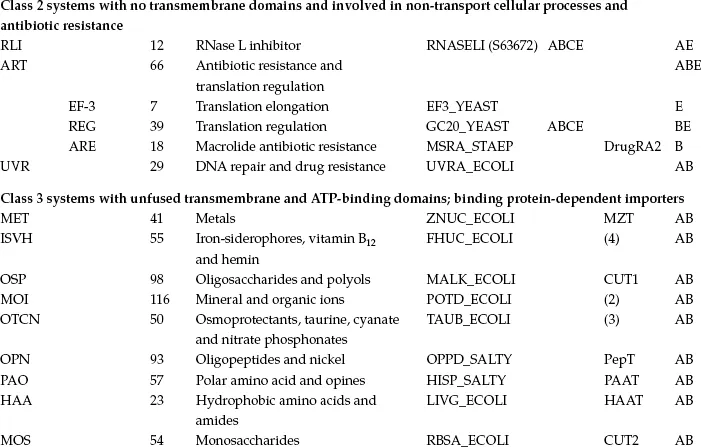
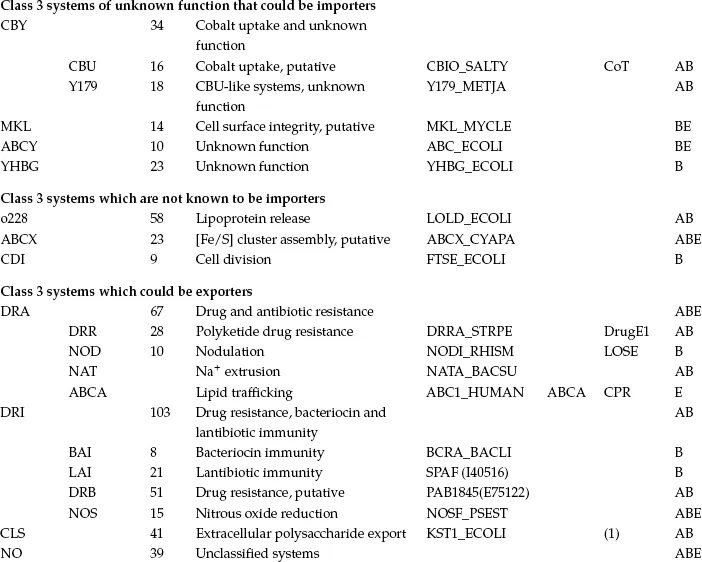
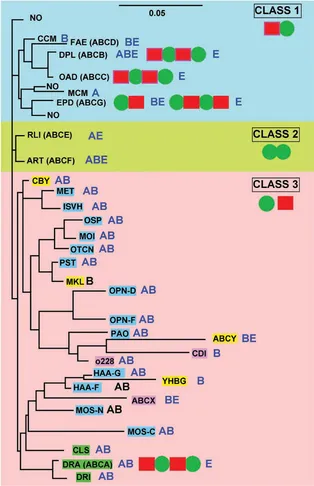
Figure 1.1 Unrooted simplified phylogenetic tree of ABC proteins and domains. For the sake of clarity, only the branches pointing to families have been drawn. The major subdivisions of the tree are indicated according to the nomenclature used in the text. Class 1: systems with fused ABC and IM domains (exporters); class 2: systems with no known transmembrane domains (antibiotic resistance, translation, etc.); class 3: systems with IM and ABC domains carried by independent polypeptide chains (BPD importers and other systems). Under the name of the class, the minimal consensus organization of ABC systems is represented by colored symbols in a linear fashion. IM proteins or domains are represented by red rectangles and ABC proteins or domains by green circles. When the organization of a system in a family does not fit exactly with the consensus, it is indicated on the same line as the system name. In class 3, BPD transporters are highlighted in blue, while systems that are not conclusively related to import are highlighted in purple; systems that could be importers are colored in yellow and systems that could be exporters in green. The sequences of UVR family proteins were omitted from this analysis (see the section on the UVR family for details). Family names are abbreviated according to the conventions used in Table 1.1 and throughout the text and the nomenclature of human ABC systems is given in parentheses after the name of the family. NO represents a few sequences with unknown function and apparently unrelated to neighboring families. They are not discussed in the text. OPN-D, OPN-F; HAA-F, HAA-G and MOS-N, MOS-C correspond to the two different ABC subunits of OPN, HAA and MOS systems, respectively. The distribution of the systems in the three kingdoms of life is indicated as follows: A (archaea), B (bacteria) and E (eukaryotes). The scale at the top of the figure corresponds to 5% divergence per site between sequences.
FAMILIES OF ABC SYSTEMS IN LIVING ORGANISMS
This classification was derived solely on the basis of the comparison of the s...