
eBook - ePub
Intermolecular and Surface Forces
Jacob N. Israelachvili
This is a test
Buch teilen
- 710 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Intermolecular and Surface Forces
Jacob N. Israelachvili
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
This reference describes the role of various intermolecular and interparticle forces in determining the properties of simple systems such as gases, liquids and solids, with a special focus on more complex colloidal, polymeric and biological systems. The book provides a thorough foundation in theories and concepts of intermolecular forces, allowing researchers and students to recognize which forces are important in any particular system, as well as how to control these forces. This third edition is expanded into three sections and contains five new chapters over the previous edition.
- Starts from the basics and builds up to more complex systems
- Covers all aspects of intermolecular and interparticle forces both at the fundamental and applied levels
- Multidisciplinary approach: bringing together and unifying phenomena from different fields
- This new edition has an expanded Part III and new chapters on non-equilibrium (dynamic) interactions, and tribology (friction forces)
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Intermolecular and Surface Forces als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Intermolecular and Surface Forces von Jacob N. Israelachvili im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Sciences biologiques & Biologie moléculaire. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
1 Historical Perspective
1.1 The Four Forces of Nature
It is now well-established that there are four distinct forces in nature. Two of these are the strong and weak interactions that act between neutrons, protons, electrons, and other elementary particles. These two forces have a very short range of action, less than 10−5 nm, and belong to the domain of nuclear and high-energy physics. The other two forces are the electromagnetic and gravitational interactions that act between atoms and molecules (as well as between elementary particles). These forces are effective over a much larger range of distances, from subatomic to practically infinite distances, and are consequently the forces that govern the behavior of everyday things (Figure 1.1). For example, electromagnetic forces—the source of all intermolecular interactions—determine the properties of solids, liquids, and gases, the behavior of particles in solution, chemical reactions, and the organization of biological structures. Gravitational forces account for tidal motion and many cosmological phenomena, and when acting together with intermolecular forces, they determine such phenomena as the height that a liquid will rise in small capillaries and the maximum size that animals and trees can attain (Thompson, 1968).
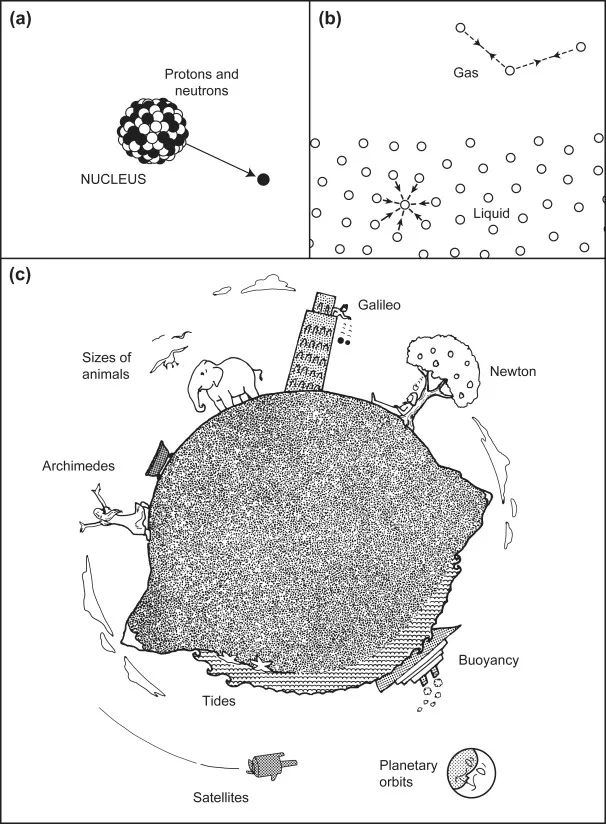
Figure 1.1 The forces of nature. (a) Strong nuclear interactions hold protons and neutrons together in atomic nuclei. Weak interactions are involved in electron emission (β decay). (b) Electrostatic (intermolecular) forces determine the cohesive forces that hold atoms and molecules together in solids and liquids. (c) Gravitational forces affect tides, falling bodies, and satellites. Gravitational and intermolecular forces acting together determine the maximum possible sizes of buildings, mountains, trees, and animals.
This book is mainly concerned with intermolecular forces. Let us enter the subject by briefly reviewing its historical developments from the ancient Greeks to the present day.
1.2 Greek and Medieval Notions of Intermolecular Forces
The Greeks were the first to consider forces in a nonreligious way (see inset on the front cover and a brief explanation on the inside of the cover page). They found that they needed only two fundamental forces to account for all natural phenomena: Love and Hate. The first brought things together, while the second caused them to part. The idea was first proposed by Empedocles around 450 B.C., was much “improved” by Aristotle, and formed the basis of chemical theory for 2000 years.
The ancients appear to have been particularly inspired by certain mysterious forces, or influences, that sometimes appeared between various forms of matter (forces that we would now call magnetic or electrostatic). They were intrigued by the “action-at-a-distance” property displayed by these forces, as well as by gravitational forces, and they were moved to reflect upon their virtues. What they lacked in concrete experimental facts they more than made up for by the abundant resources of their imagination (Verschuur, 1993). Thus, magnetic forces could cure diseases, though they could also cause melancholy and thievery. Magnets could be used to find gold, and they were effective as love potions and for testing the chastity of women. Unfortunately, some magnetic substances lost their powers if rubbed with garlic (but they usually recovered when treated with goat’s blood). Electric phenomena were endowed with attributes no less spectacular, manifesting themselves as visible sparks in addition to a miscellany of attractive or repulsive influences that appeared when different bodies were rubbed together. All these wondrous practices, and much else, were enjoyed by our forebears until well into the seventeenth century and may be said to constitute the birth of our subject at the same time as alchemy, astrology, and the search for perpetual motion machines, which paved the way for chemistry and physics.
Still, notwithstanding the unscientific or prescientific nature of these practices, some important conceptual breakthroughs were made that deserve to be recognized. Ask any schoolboy or schoolgirl what the three most memorable scientific discoveries of antiquity are, and they will very likely mention Archimedes, Galileo, and Newton (see Figure 1.1). In each case, what happened (or is alleged to have happened) is well known, but what is less well known are the conceptual breakthroughs whose implications are with us today. Put into modern jargon, when Archimedes (287–212 B.C.) discovered Archimedes’ Principle, he had discovered that the force of gravity on a body can change—even its sign from attraction to repulsion—when it is placed in a medium that it displaces. Later we shall see that such displacement effects occur with many other types of interactions, such as van der Waals forces, with important consequences that in some cases have only recently been appreciated.
1.3 The Seventeenth Century: First Scientific Period
The notion of doing experiments to find out how nature works was an unknown concept until Galileo (1564–1642) demonstrated its power in his classic experiments on gravity, the motion of bodies, optics, astronomy, and proving the existence of a vacuum. In this he introduced the modern “scientific method” (Table 1.1).
Table 1.1 Scientists Who Made Major Contributions to Our Understanding of Intermolecular Forces (including some whose contribution was indirect)
But Galileo also introduced a new way of thinking that was not purely metaphysical. As an example, his experiment of throwing two different balls from the Tower of Pisa was conducted only after he had worked out the answer by applying a new form of reasoning—what we would today call “scaling arguments”—to Aristotelean Physics (see Problem 1.1), and his testing of his hypothesis by direct experiment was at the time also highly novel.1 This is also true of Newton’s discovery of the Law of Gravitation when an apple fell on his head. We must pause to think about how many previous heads, when struck by a falling apple, were prompted to generalize the phenomenon to other systems such as the orbit of the moon around the earth when acted on by the same force—a thought process that requires a leap of the imagination by a factor of 106 in time, 108 in distance, and 1024 in mass.
T...