Recent Developments and Perspectives on Solar-driven Fine Chemicals Synthesis: From the Reaction System to 2D Photocatalysts
1.1 Introduction
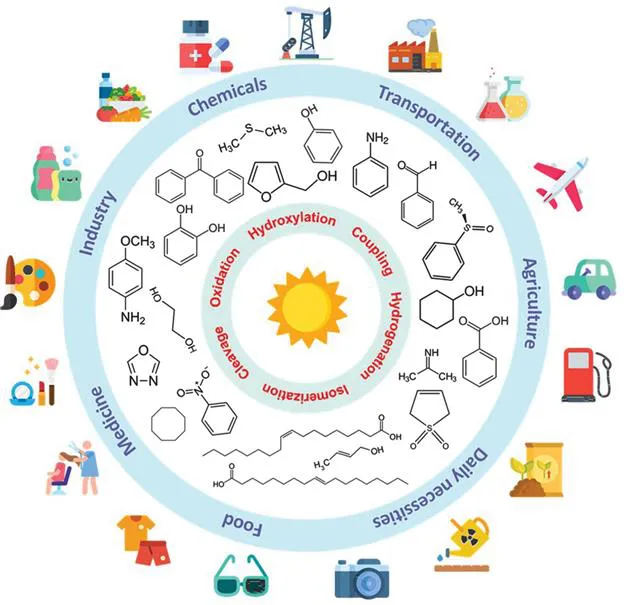
Fine chemicals are complex, single and pure chemicals produced in limited quantities by a multi-step batch chemical or biological process (from Wikipedia, 2021, https://en.wikipedia.org/wiki/Fine_chemical). In most cases, the production of these fine chemicals is limited (<1000 tons per year)under strict specifications, leading to high prices (>$10 kg−1). These high value-added products can be used as the starting materials for speciality chemicals such as agrochemicals and pharmaceuticals (Figure 1.1). Fine chemicals have become a significant part of the chemical industry since the late 1970s. However, so far, most of their production on the industrial scale is operated under high temperature and high pressure, resulting in environmental pollution and high energy consumption. In this regard, photo(electro)catalysis enables a more sustainable and economical approach by virtue of the solar energy used. At present, many photocatalytic reaction systems using semiconductors have been proven to be feasible under mild conditions. Simple processes, green reaction conditions and renewable solar energy bring promising prospects for photocatalyst applications in CO2 reduction, water splitting to produce clean H2, fine chemicals synthesis, etc. However, the low conversion and selectivity still hinder the popularisation and the industrial application of photocatalytic technology.1 In recent years, great progress has been made in heterogeneous photocatalyst synthesis for enhanced catalytic activity under visible light irradiation.2 By manipulating the morphology of heterogeneous catalysts, nanoscale photocatalysts with higher intrinsic activity, larger specific surface areas and more active sites can be synthesised. Among them, two-dimensional (2D) nanomaterials have attracted special research interest due to their unique structural advantages and excellent photocatalytic performance.
Figure 1.1 Reactions to synthesise fine chemicals and their corresponding applications in industry and modern life. Icons made by Freepik, Smashicons, Pixel perfect, Flat Icons and photo3idea_studio from www.flaticon.com.
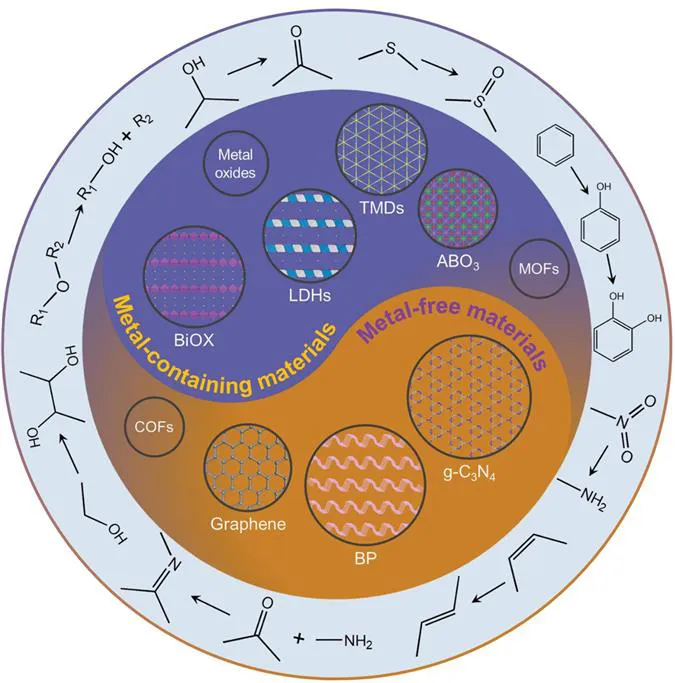
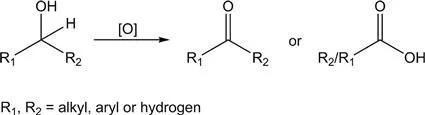
2D nanomaterials can be nanoscale thick or even as thin as one atom.3 The family of 2D nanomaterials include graphene,4–8 graphitic carbon nitride (g-C3N4),9–11 transition metal dichalcogenides (TMDs),12–15 layered double hydroxides (LDHs),16,17 black phosphorus (BP),18,19 MXenes,20 hexagonal boron nitride (h-BN),21–23 perovskites,24–27 metal–organic frameworks (MOFs),28–30 covalent–organic frameworks (COFs)31 and other sheet-like materials32–41 (Figure 1.2). Because electrons are confined to a two-dimensional scale, 2D materials have unique physical and chemical properties, such as excellent electrical conductivities, carrier mobility, etc.42 The nanosheet structure and atomic-level thickness endow 2D materials with high specific surface area and full exposure of the surface active sites, thus showing high catalytic performance in photo(electro)catalysis such as selective oxidation of alcohols, hydrogenation, and C–C and C–N coupling that will be discussed later.43
Figure 1.2 Types of 2D materials and crystal structures of several typical 2D materials for efficient fine chemicals synthesis.
Among the metal-free 2D materials, g-C3N4 has the advantages of low cost, easy synthesis, unique band structure and broad adsorption in the visible light range. As a visible-light-responsive photocatalyst, it has been widely studied in the field of photocatalysis.44 To enhance its photocatalytic performance, researchers have been modifying and optimising g-C3N4 through element doping,45,46 morphology regulation47,48 and heterogeneous junction construction.49,50 Different from g-C3N4 and graphene, BP has a wide band response and shows a strong light conduction efficiency. BP is a natural p-type semiconductor with a direct bandgap, which can be adjusted by changing the number of stacked phosphorus layers, and has significant anisotropy. Among the two-dimensional materials, BP has high electron mobility, and its optical and photoelectric properties have greater advantages for further photocatalysis.51–53
Layered double hydroxides (LDHs) are a kind of layered clay material, and their structure can be optimised by adjusting the type and proportion of metal elements for enhanced catalytic performance. Through the calcination strategy, LDHs can be further transformed into mixed-metal oxides (MMO) or metal-supported materials, which expose more active sites and improve catalytic performance.54,55 Similarly, TMDs,56,57 bismuth oxyhalides (BiOX),58,59 perovskite (ABO3)60,61 and metal-containing layered materials62–65 are composed of polyhedral coordination units of metallic and non-metallic elements. They can be modified by controlling the morphology, elements and crystal surface, and constructing a heterojunction to improve the exposure of active elements or defects, enhance the light absorption capacity of materials and the separation and transfer efficiency of photogenerated carriers, change the adsorption ability of reactants and products, and improve the conversion rate and selectivity of photocatalytic reactions. Herein, this review summarises the recent development of 2D materials in the photocatalytic synthesis of fine chemicals and biomass valorisation, such as selective oxidation, C–C/C–N coupling, hydrogenation, cis–trans isomerisation and C–C/C–O cleavage, etc. In order to facilitate the reader's understanding, a brief history of the development of some common fine chemicals synthesis reactions is also provided.