M. Eggena and J. Schindler,b
1.1 Introduction
Fifty five years ago, Max Perutz and John Kendrew received the 1962 Nobel Prize in Chemistry. Their pioneering work delivered the first 6 Å structure of the protein Myoglobin, and represented the foundation for modern structural biology.1–3 This first atomic-level picture heralded the complexity of the protein universe and provoked questions about how to predict the driving forces that allow proteins to rapidly fold into biologically relevant conformations. This was the beginning of biophysics. In 1969, the first enzyme structure of the extracellular nuclease of Staphylococcus Aureus as an enzyme-inhibitor complex was solved by F. Albert Cotton and coworkers.4
Thus began significant motivation to devise and evolve computational methods to predict protein folding. “Force fields” in modern computer simulation draw their information primarily from the ∼40 000 protein families represented in the Protein Data Bank (PDB), where structures obtained by X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy and, increasingly, cryo-electron microscopy (cryo-EM) are deposited. Today, X-ray crystallography has been extended further to characterise inherently disordered proteins and protein aggregation.5–8
Analysis of structural information has been applied to study protein equilibria and dynamics. The driving forces of (1) hydrogen bonding, (2) van der Waals interactions, (3) backbone conformational preferences, (4) electrostatics, and (5) hydrophobic interactions leading to the precise folding of proteins produce the minimum energy conformation, as can be seen crystallographically.9 Beyond the minimum energy conformation determined through x-ray crystallography, additional lower energy minima representing the dynamic movement of proteins can be studied by other biophysical techniques such as protein NMR and proton deuterium exchange mass spectrometry (HDX-MS).
Small molecules must be identified and optimized to productively interact with the protein's disease-relevant conformation or surface. This requires several pieces of information: (1) disease biology, (2) an understanding of the protein form or forms within the cell, and (3) an understanding of the protein surface and the potential interactions and MOA that can occur with the small molecule. Many of the same forces that impact protein conformation influence the low energy conformations and physical properties of drugs and can be assessed through small molecule crystallography, analytical methods and computational minimizations. Ultimately, the small molecule design team, the chemist, computational scientist, and structural biologist are challenged to identify opportunities for optimal molecular recognition from the protein. Optimization requires an understanding of interaction geometries and approximate contributions identified through crystal structures and demonstrated through affinity data.10 However, molecular interactions behave in a highly “non-additive fashion” and are context-dependent. Solvation of the protein and small molecule, long-range interactions and conformational changes of the protein (see Figure 3, ref. 9) all influence binding energetics.
Design of the small molecule must focus on specific intermolecular reactions based on available structural information. The most important and well defined are hydrogen bond interactions directly with the protein or through structural water.11–13 Weaker hydrogen bonds are also sometimes available when an aromatic ring can act as H-bond acceptor.14 Although H-bonds are among the strongest, these interactions should not be the sole focus of the designer. Orthogonal multipolar interactions of C–F to C=O such as the Bürgi–Dunitz angle,15 halogen bonds that leverage sigma-hole anisotropy, and cation–Pi interactions are also opportunities in the design of a molecule.16
Ultimately, the amount of hydrophobic surface buried through Van der Waals interactions upon ligand binding appears to best correlate with binding affinity.17 This concept holds for a diverse set of protein–ligand complexes, including protein–protein interactions.18 All other types of interactions are highly context-dependant and their utility must be tested within a given target. The thermodynamics and kinetics of the target interactions can be evaluated to understand progress and will be discussed in a later section.
Intramolecular interactions within a protein as well as stabilizing intermolecular interactions with an inhibitor were key to delivering the first biophysical technique, X-ray crystallography. The power of interpreting a low-energy atomic-level picture of a molecule bound to an enzyme created a paradigm shift in drug discovery. However, the dynamic nature of proteins requires an understanding of both their minimum energy states and also the status of their movement in other conformations. Further, these states must be measured and interpreted amidst the complex and dynamic processes in the cell. Techniques such as molecular imaging can provide direct measurement of the location and progression of processes. Connecting these techniques to non-invasive measurements of patient disease pathology and response in the evaluation of neurodegeneration,19 cardiovascular disease20 and oncology21,22 can leverage molecular imaging through PET-CT and MRI scans.
Biophysics is utilized throughout drug discovery strategies from target identification and selection, construct selection, screening, ligand optimization, drug development and ultimately imaging within our patients. The following sections will provide context for the techniques presented in this book.
1.2 Evolution of Biophysics in Medicinal Chemistry
1.2.1 Phenotypic Drug Discovery
The earliest drug identification and approval were driven by small molecule phenotypic drug discovery (PDD) approaches.23–25 This strategy involved identifying and optimizing efficacy in animal physiological and behavioural models for human diseases, and ex vivo tissue-based or phenotypic cellular assays. While many drugs used clinically today were discovered agnostic of their target protein, work in this fashion increased the necessary investment in the systematic iteration and synthesis of lead molecules focusing on potency and phenotype. Without specific target and mechanism of action information, agents often displayed poly-pharmacology, which could be good for disease modification or bad for toxicology. Coupled with extended times in early stage discovery, the advent of protein crystallography, and the potential for off-target liabilities that wouldn't be identified until entry into the clinic, drug discovery attempted to minimize these challenges through targeted therapies, that is, target directed drug discovery (TDD) (Figure 1.1).26
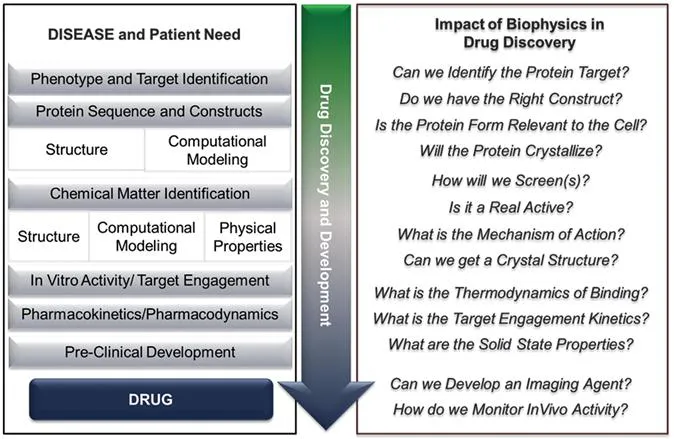
Figure 1.1 Drug discovery pathway to a medicine with examples of key questions that biophysics can address experimentally and influence in molecule identification, progression and design.
1.2.2 Targeted and Fragment-based Drug Discovery
With the advent of a deeper understanding of the proteins involved in a disease pathway through cell biology tools such as siRNA, proteomics, knock-outs, and human genetics (mutations found in patient populations), drug discovery efforts have focused more on specific proteins believed to be involved in a disease. This knowledge has led to a paradigm shift in drug discovery that went from dosing a limited set of compounds in animal models to screening 250 000 to 1 million compounds in biochemical assays in a specific protein. Typically, the biochemical assays were performed in formats that looked at competition binding or enzyme activity against recombinant proteins.
Drug discovery targets, in response, moved into more complex protein targets such has epigenetics, protein–protein interactions (PPI), membrane-bound proteins and transporters, and protein aggregation. This resulted in gaps or greater challenges in screening approaches, and protein expression, production and purification. The new target space ultimately affected structural biology approaches. As an example, discrete protein target EZH2 (Section 1.5.2), actually a component of a complex of proteins, requires this complex for activity and stability. In addition, protein complexes could in theory be inhibited by binding in different proteins and read-out as competitive, non-competitive, uncompetitive, mixed or irreversible in nature. In the case of protein–protein interactions, one may simply be interacting with a protein and disrupting a productive, functional interaction with a second protein.
This paradigm shift in targets also resulted in re-thinking how compound libraries were computationally selected for screening. For example, PPI targets required larger molecules that were not present in most traditional libraries based on Lipinki's rules,24 and when starting points were found in traditional screening, there was very little room to maintain potency while fixing the physical properties. To overcome gaps in compound collections and property challenges, some drug discovery groups implemented a new strategy and proposed to screen compound “fragments” with MW<300 Da. Fragment-based drug discovery (FBDD) takes advantage of Jencks’ description of Gibb's free energy and attributes of the binding energy between enzyme and substrate.25 By beginning with fragments, a greater chemical space could be sampled with smaller sets of molecules while optimizing biological activity and physical properties in parallel.26 This introduced a strategic shift in how to approach drug discovery and an opportunity to identify chemical matter without a large compound collection.
The challenge with FBDD is that the initial compound actives have highly efficient per heavy atom (non-hydrogen) target binding but low measured binding constants. Given their low affinity, high concentration biochemical assays (enzyme or competition binding) were used, but did not behave or were still not sensitive enough to pick up fragments in all targets or target classes. Sensitive biophysical methods had to be identified. Teams also needed orthogonal methods to confirm the fragment as a legitimate active, identify a potential MOA, and to provide clues about vectors in order to decorate the molecule for efficient, key interactions within the larger protein active site.
1.2.3 Phenotypic Drug Discovery 2.0
A renewed interest in PDD strategies was influenced by a focus on the complexity of patients and their disease states in oncology, autoimmune disease and neurodegeneration. Coupled with technological developments at the interfaces of disciplines scientists have been able to deliver complex cell culture systems and gene-edited disease relevant mutations for phenotypic screening assays.27–29 Advances in mass spectrometry methods such as affinity selection (AS-MS), cellular thermal shift assays (CETSA) and cDNA expression microarray technologies have allowed probing of complex pathways. Improvements in cell-based and model-organism based automated screens,30–32 along with the development of new biophysical methodologies have enabled improved screening, faster identification of targets and profiling of the MOA in complex biological samples at the genomic, proteomic and phenotypic levels.33
1.2.4 Evolving Compound Collections and Chemical Technologies
Highly elaborate compounds that became part of compound collections were de...