
eBook - ePub
Cellulose Science and Technology
Chemistry, Analysis, and Applications
Thomas Rosenau, Antje Potthast, Johannes Hell, Thomas Rosenau, Antje Potthast, Johannes Hell
This is a test
Buch teilen
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Cellulose Science and Technology
Chemistry, Analysis, and Applications
Thomas Rosenau, Antje Potthast, Johannes Hell, Thomas Rosenau, Antje Potthast, Johannes Hell
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
This book addresses both classic concepts and state-of-the-art technologies surrounding cellulose science and technology. Integrating nanoscience and applications in materials, energy, biotechnology, and more, the book appeals broadly to students and researchers in chemistry, materials, energy, and environmental science. • Includes contributions from leading cellulose scientists worldwide, with five Anselm Payen Cellulose Award winners and two Hayashi Jisuke Cellulose Award winners
• Deals with a highly applicable and timely topic, considering the current activities in the fields of bioeconomies, biorefineries, and biomass utilization
• Maximizes readership by combining fundamental science and application development
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Cellulose Science and Technology als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Cellulose Science and Technology von Thomas Rosenau, Antje Potthast, Johannes Hell, Thomas Rosenau, Antje Potthast, Johannes Hell im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Technology & Engineering & Materials Science. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
1
Aminocelluloses – Polymers with Fascinating Properties and Application Potential
Thomas Heinze Thomas Elschner and Kristin Ganske
Centre of Excellence for Polysaccharide Research, Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University of Jena, Humboldtstraße 10, D‐07743 Jena, Germany
1.1 Introduction
Cellulose is a linear D‐glucan containing β‐1 → 4 linkages and is the world's most abundant natural polymer with an estimated annual global production of about 1.5 × 1012 tons and, hence, a very important renewable and sustainable resource [1]. Although unmodified cellulose is used largely as paper, board, and fibers, there is huge space to design novel and advanced products based on cellulose by its chemical modification. In particular, esters and ethers of cellulose are most important [1, 2].
Due to their low‐cost production, biodegradability, and low‐toxicity cationized polysaccharides are promising in fields of effluent treatment, papermaking, and food, cosmetic, pharmaceutical, petroleum, and textile industries, as well as in analytical chemistry and molecular biology [3]. In particular, cationic cellulose derivatives gain increasing interest in different scientific and industrial fields, e.g. as flocculation agents [4], being an alternative to toxic polyacrylamide. In Germany, the disposal of sludge treated with polyacrylamides has been forbidden in areas under cultivation since 2014 [5].
Considering the recent literature, the huge amount of publications was summarized in reviews about cationic synthetic polyelectrolytes [6] as well as cationized polysaccharides (amino and ammonium hydroxypropyl ethers) [3]. However, in this chapter, the authors will not review the cationic ethers; the overview refers to cationic esters, 6‐deoxy‐6‐amino cellulose derivatives, and amino carbamates of cellulose. In spite of the industrial applications that are usually associated with cationic polymers, a variety of advanced polymer coatings providing sophisticated features, e.g. biosensors or immuno assays, will be presented.
1.2 Amino‐/ammonium Group Containing Cellulose Esters
1.2.1 (3‐Carboxypropyl)trimethylammonium Chloride Esters of Cellulose
An efficient approach to cationic cellulose derivatives is the esterification of the hydroxyl groups with cationic carboxylic acids. Activated carboxylic acids such as acyl chlorides or acid anhydrides are not appropriate due to their limited solubility, availability, and the formation of acidic by‐products. However, the esterification applying imidazolides obtained from the corresponding carboxylic acid and N,N‐carbonyldiimidazole (CDI) is a mild and efficient synthesis strategy [2].
To synthesize cationic cellulose esters (3‐carboxypropyl)trimethylammonium chloride was activated with CDI in dimethylsulfoxide (DMSO) separately and allowed to react with cellulose dissolved in N,N‐dimethylacetamide (DMA)/LiCl [7]. Thus, a product with a degree of substitution (DS) of 0.75 was accessible that could be characterized by 13C NMR spectroscopy (Figure 1.1).
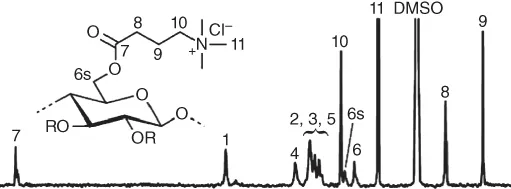
Figure 1.1 13C NMR spectrum of cellulose (3‐carboxypropyl)trimethylammonium chloride ester in DMSO‐d6.
Source: Vega et al. 2013 [7]. Reproduced with permission of American Chemical Society.
Cellulose (3‐carboxypropyl)trimethylammonium chloride esters adsorbed on cellulose films may trigger the protein adsorption, which is a key parameter in the design of advanced materials for a variety of technological fields [8]. The protein affinity to the surface can be controlled by the charge density and solubility, adjusted by the pH value, the concentration of protein and the DS of the tailored cationic cellulose derivative. To understand the influence of the cationic cellulose ester on the protein affinity, the interaction capacity with fluorescence‐labeled bovine serum albumin (BSA) at different concentrations and pH values was carried out (Figure 1.2). The adsorbed material was quantified applying QCM‐D (quartz crystal microbalance with dissipation monitoring, wet mass) and MP‐SPR (multi‐parameter surface plasmon resonance, dry mass). Thus, the amount of coupled water in the layer could be evaluated by a combination of QCM‐D and surface plasmon resonance (SPR) data. According to these studies the interaction decreases in order of pH 5 > pH 6 > pH 7 and DShigh > DSlow, respectively. The adsorption of BSA may be adjusted over a range from 0.6 to 3.9 mg m−2 (dry mass). This approach is suitable to utilize BSA as blocking agent on the surface and achieve selective functionalization of cellulosic surfaces by functional proteins (e.g. antibodies).
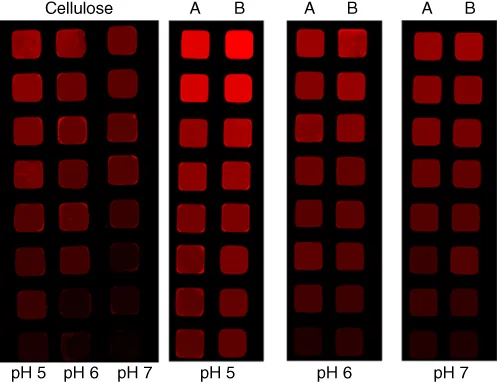
Figure 1.2 Cyclic olefin polymer slides equipped with cellulose and cellulose (3-carboxypropyl)trimethylammonium chloride ester incubated with different concentrations of labeled BSA (1000, 500, 10...