
eBook - ePub
Chemistry of Nucleic Acids
Harri Lönnberg
This is a test
Compartir libro
- 364 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Chemistry of Nucleic Acids
Harri Lönnberg
Detalles del libro
Vista previa del libro
Índice
Citas
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Chemistry of Nucleic Acids un PDF/ePUB en línea?
Sí, puedes acceder a Chemistry of Nucleic Acids de Harri Lönnberg en formato PDF o ePUB, así como a otros libros populares de Biological Sciences y Biochemistry. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
1 Nucleosides: structure, nomenclature and solution equilibria
1.1 Nomenclature
Nucleosides, the monomeric constituents of nucleic acids, are N-glycosylated derivatives of two different categories of heteroaromatic nitrogen bases, namely monocyclic pyrimidines and bicyclic purines. The pyrimidine bases are cytosine, uracil and thymine, and the purine bases are adenine and guanine. Uracil occurs only in RNA and thymine in DNA, while the other bases are common for both types of nucleic acids. The structures and enumeration of these canonical nucleic acid bases and nucleosides are depicted in Figure 1.1. As indicated, the glycosyl group is attached to N1 of pyrimidine bases and N9 of purine bases. The enumeration of the glycosyl moiety starts from the anomeric carbon, that is, the carbon atom bound to the nucleobase, not from the ring oxygen. In ribonucleosides (constituents of RNA), the glycosyl moiety is β-d-ribofuranosyl group and in 2′-deoxyribonucleosides (constituents of DNA) 2-deoxy-β-d-erythro-pentofuranosyl group. The latter group is often called 2-deoxy-β-d-ribofuranosyl group, but this name is not consistent with the nomenclature of carbohydrates [1]. The prefix “ribo” refers to a sugar having three stereogenic centers in addition to the anomeric (C1′) carbon. 2-Deoxypentoses contain only two nonanomeric stereogenic centers; hence, the correct prefixes are “erythro” and “threo.” The names of ribonucleosides are derived from the names of their base moieties: adenosine, guanosine, cytidine and uridine. The names of 2′-deoxyribonucleosides have, in turn, been formed from the names of the respective ribonucleosides by a prefix 2′-deoxy, with the exception of thymine derivative. This 2′-deoxyribonucleoside is for historical reasons called just thymidine.
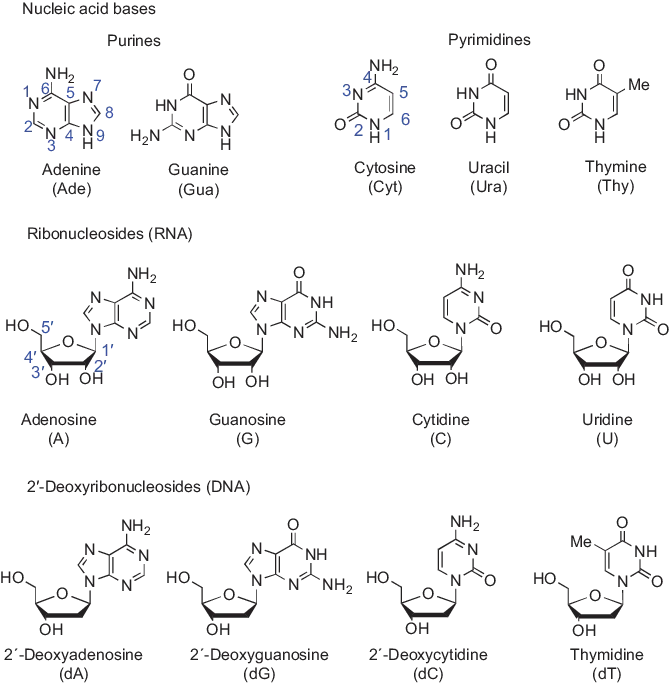
Figure 1.1: The structure and enumeration of nucleic acid bases, ribonucleosides and 2′-deoxyribonucleosides.
The names of substituted or modified nucleosides are derived from the names of the parent nucleosides, as exemplified by a few illustrative examples in Figure 1.2. Substituents on the sugar and base moiety of nucleosides are indicated in the beginning of the name in alphabetical order. A missing ring nitrogen is indicated by a prefix “deaza” and an extra ring nitrogen by a prefix “aza.” One should, however, note that this kind of nomenclature is applicable only as long as the sugar moiety is a five-membered d-sugar having a ribo (for ribonucleosides) or erythro configuration (for 2′-deoxyribonucleosides) and the anomeric configuration is β. If the configuration of the sugar moiety, enantiomeric form or ring size, is changed, the name of the nucleoside is formed by adding the name of the sugar moiety as a substituent to the name of the base moiety. This is also the case when the base moiety is heavily modified. The compound is then named as a glycosylated heterocyclic compound. Sometimes abbreviations such as ara-, lyxo- or xylo-adenosine are used. This means that the sugar moiety still is a β-d-glycofuranosyl group, but the configuration is not any more ribo. In case the sugar ring has been opened by cleaving a C–C bond, the site of the missing bond is indicated by a prefix “seco,” for example, 2′,3′-seco-adenosine. In addition, some noncanonical nucleobases and nucleosides have trivial names that are commonly used. The N2-deamino analogs of guanine and guanosine are called hypoxanthine and inosine, respectively, and the 2-oxo derivatives of these are known as xanthine and xanthosine.
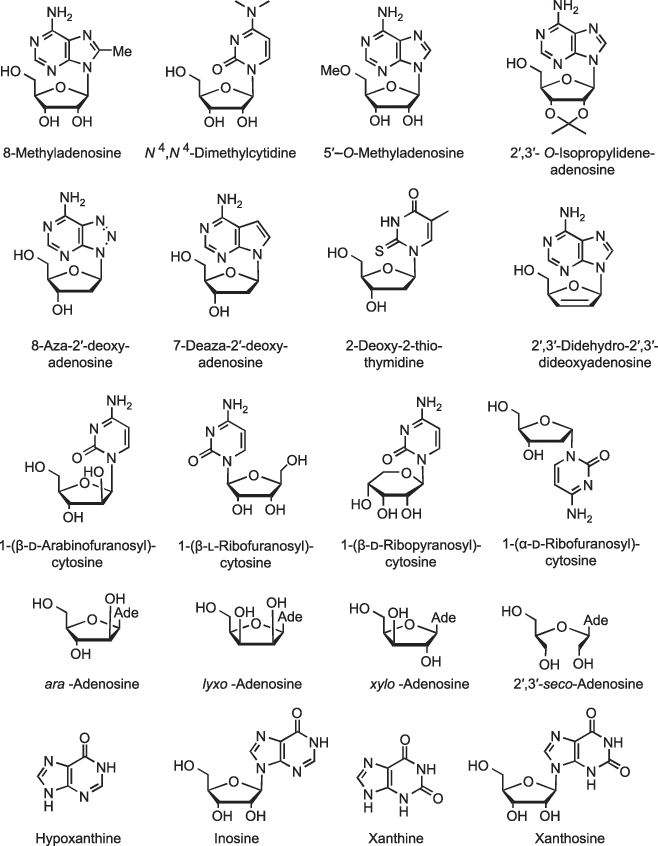
Figure 1.2: Examples of the names of substituted or modified nucleosides.
1.2 Cyclonucleosides
Cyclonucleosides are synthetic analogs of nucleosides having an additional covalent linkage between the sugar and base moiety. Only one cyclonucleoside, 3,5′-anhydro-xanthosine, has bee...