
eBook - ePub
Chemistry of Nucleic Acids
Harri Lönnberg
This is a test
Partager le livre
- 364 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Chemistry of Nucleic Acids
Harri Lönnberg
Détails du livre
Aperçu du livre
Table des matières
Citations
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Chemistry of Nucleic Acids est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Chemistry of Nucleic Acids par Harri Lönnberg en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Biological Sciences et Biochemistry. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
1 Nucleosides: structure, nomenclature and solution equilibria
1.1 Nomenclature
Nucleosides, the monomeric constituents of nucleic acids, are N-glycosylated derivatives of two different categories of heteroaromatic nitrogen bases, namely monocyclic pyrimidines and bicyclic purines. The pyrimidine bases are cytosine, uracil and thymine, and the purine bases are adenine and guanine. Uracil occurs only in RNA and thymine in DNA, while the other bases are common for both types of nucleic acids. The structures and enumeration of these canonical nucleic acid bases and nucleosides are depicted in Figure 1.1. As indicated, the glycosyl group is attached to N1 of pyrimidine bases and N9 of purine bases. The enumeration of the glycosyl moiety starts from the anomeric carbon, that is, the carbon atom bound to the nucleobase, not from the ring oxygen. In ribonucleosides (constituents of RNA), the glycosyl moiety is β-d-ribofuranosyl group and in 2′-deoxyribonucleosides (constituents of DNA) 2-deoxy-β-d-erythro-pentofuranosyl group. The latter group is often called 2-deoxy-β-d-ribofuranosyl group, but this name is not consistent with the nomenclature of carbohydrates [1]. The prefix “ribo” refers to a sugar having three stereogenic centers in addition to the anomeric (C1′) carbon. 2-Deoxypentoses contain only two nonanomeric stereogenic centers; hence, the correct prefixes are “erythro” and “threo.” The names of ribonucleosides are derived from the names of their base moieties: adenosine, guanosine, cytidine and uridine. The names of 2′-deoxyribonucleosides have, in turn, been formed from the names of the respective ribonucleosides by a prefix 2′-deoxy, with the exception of thymine derivative. This 2′-deoxyribonucleoside is for historical reasons called just thymidine.
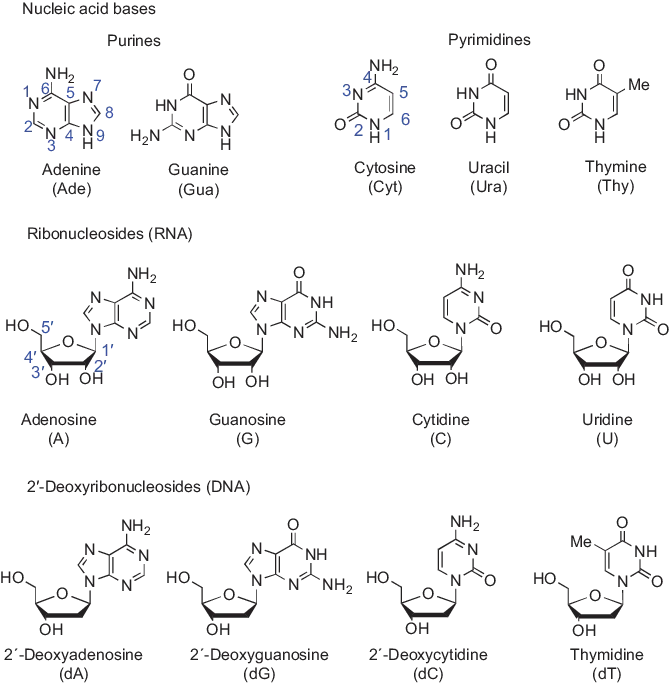
Figure 1.1: The structure and enumeration of nucleic acid bases, ribonucleosides and 2′-deoxyribonucleosides.
The names of substituted or modified nucleosides are derived from the names of the parent nucleosides, as exemplified by a few illustrative examples in Figure 1.2. Substituents on the sugar and base moiety of nucleosides are indicated in the beginning of the name in alphabetical order. A missing ring nitrogen is indicated by a prefix “deaza” and an extra ring nitrogen by a prefix “aza.” One should, however, note that this kind of nomenclature is applicable only as long as the sugar moiety is a five-membered d-sugar having a ribo (for ribonucleosides) or erythro configuration (for 2′-deoxyribonucleosides) and the anomeric configuration is β. If the configuration of the sugar moiety, enantiomeric form or ring size, is changed, the name of the nucleoside is formed by adding the name of the sugar moiety as a substituent to the name of the base moiety. This is also the case when the base moiety is heavily modified. The compound is then named as a glycosylated heterocyclic compound. Sometimes abbreviations such as ara-, lyxo- or xylo-adenosine are used. This means that the sugar moiety still is a β-d-glycofuranosyl group, but the configuration is not any more ribo. In case the sugar ring has been opened by cleaving a C–C bond, the site of the missing bond is indicated by a prefix “seco,” for example, 2′,3′-seco-adenosine. In addition, some noncanonical nucleobases and nucleosides have trivial names that are commonly used. The N2-deamino analogs of guanine and guanosine are called hypoxanthine and inosine, respectively, and the 2-oxo derivatives of these are known as xanthine and xanthosine.
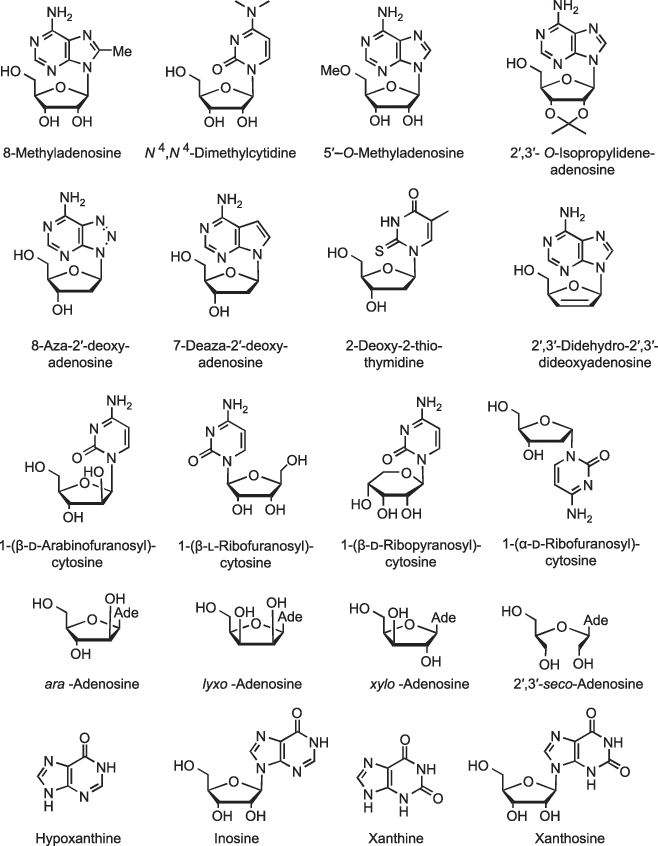
Figure 1.2: Examples of the names of substituted or modified nucleosides.
1.2 Cyclonucleosides
Cyclonucleosides are synthetic analogs of nucleosides having an additional covalent linkage between the sugar and base moiety. Only one cyclonucleoside, 3,5′-anhydro-xanthosine, has bee...