
eBook - ePub
Chemistry of Nucleic Acids
Harri Lönnberg
This is a test
Share book
- 364 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemistry of Nucleic Acids
Harri Lönnberg
Book details
Book preview
Table of contents
Citations
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Chemistry of Nucleic Acids an online PDF/ePUB?
Yes, you can access Chemistry of Nucleic Acids by Harri Lönnberg in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biochemistry. We have over one million books available in our catalogue for you to explore.
Information
1 Nucleosides: structure, nomenclature and solution equilibria
1.1 Nomenclature
Nucleosides, the monomeric constituents of nucleic acids, are N-glycosylated derivatives of two different categories of heteroaromatic nitrogen bases, namely monocyclic pyrimidines and bicyclic purines. The pyrimidine bases are cytosine, uracil and thymine, and the purine bases are adenine and guanine. Uracil occurs only in RNA and thymine in DNA, while the other bases are common for both types of nucleic acids. The structures and enumeration of these canonical nucleic acid bases and nucleosides are depicted in Figure 1.1. As indicated, the glycosyl group is attached to N1 of pyrimidine bases and N9 of purine bases. The enumeration of the glycosyl moiety starts from the anomeric carbon, that is, the carbon atom bound to the nucleobase, not from the ring oxygen. In ribonucleosides (constituents of RNA), the glycosyl moiety is β-d-ribofuranosyl group and in 2′-deoxyribonucleosides (constituents of DNA) 2-deoxy-β-d-erythro-pentofuranosyl group. The latter group is often called 2-deoxy-β-d-ribofuranosyl group, but this name is not consistent with the nomenclature of carbohydrates [1]. The prefix “ribo” refers to a sugar having three stereogenic centers in addition to the anomeric (C1′) carbon. 2-Deoxypentoses contain only two nonanomeric stereogenic centers; hence, the correct prefixes are “erythro” and “threo.” The names of ribonucleosides are derived from the names of their base moieties: adenosine, guanosine, cytidine and uridine. The names of 2′-deoxyribonucleosides have, in turn, been formed from the names of the respective ribonucleosides by a prefix 2′-deoxy, with the exception of thymine derivative. This 2′-deoxyribonucleoside is for historical reasons called just thymidine.
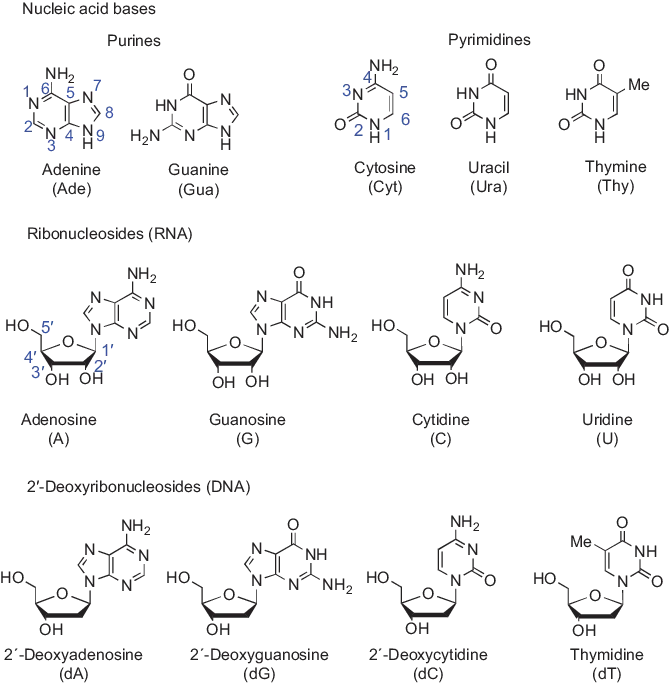
Figure 1.1: The structure and enumeration of nucleic acid bases, ribonucleosides and 2′-deoxyribonucleosides.
The names of substituted or modified nucleosides are derived from the names of the parent nucleosides, as exemplified by a few illustrative examples in Figure 1.2. Substituents on the sugar and base moiety of nucleosides are indicated in the beginning of the name in alphabetical order. A missing ring nitrogen is indicated by a prefix “deaza” and an extra ring nitrogen by a prefix “aza.” One should, however, note that this kind of nomenclature is applicable only as long as the sugar moiety is a five-membered d-sugar having a ribo (for ribonucleosides) or erythro configuration (for 2′-deoxyribonucleosides) and the anomeric configuration is β. If the configuration of the sugar moiety, enantiomeric form or ring size, is changed, the name of the nucleoside is formed by adding the name of the sugar moiety as a substituent to the name of the base moiety. This is also the case when the base moiety is heavily modified. The compound is then named as a glycosylated heterocyclic compound. Sometimes abbreviations such as ara-, lyxo- or xylo-adenosine are used. This means that the sugar moiety still is a β-d-glycofuranosyl group, but the configuration is not any more ribo. In case the sugar ring has been opened by cleaving a C–C bond, the site of the missing bond is indicated by a prefix “seco,” for example, 2′,3′-seco-adenosine. In addition, some noncanonical nucleobases and nucleosides have trivial names that are commonly used. The N2-deamino analogs of guanine and guanosine are called hypoxanthine and inosine, respectively, and the 2-oxo derivatives of these are known as xanthine and xanthosine.
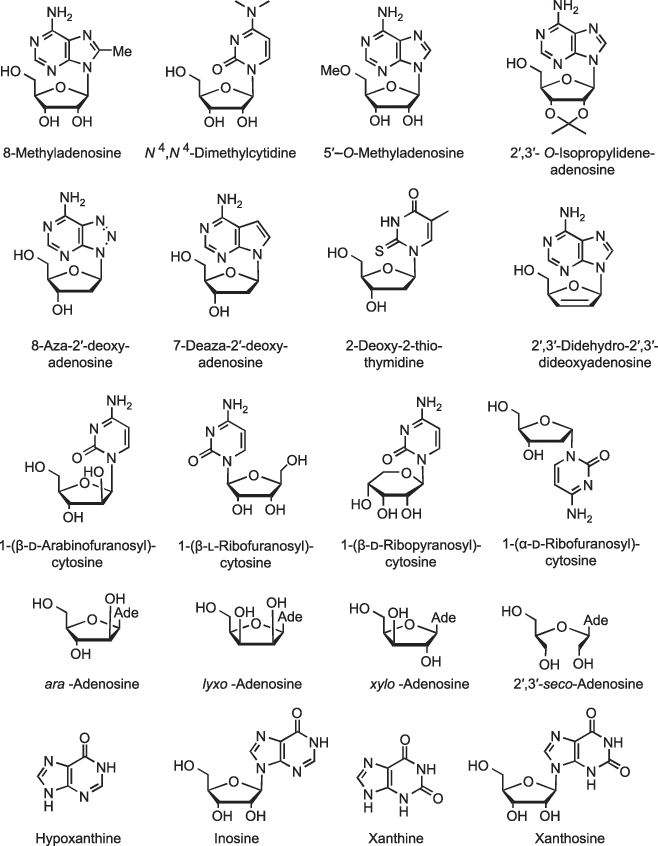
Figure 1.2: Examples of the names of substituted or modified nucleosides.
1.2 Cyclonucleosides
Cyclonucleosides are synthetic analogs of nucleosides having an additional covalent linkage between the sugar and base moiety. Only one cyclonucleoside, 3,5′-anhydro-xanthosine, has bee...