
eBook - ePub
Fundamentals of Perovskite Oxides
Synthesis, Structure, Properties and Applications
Gibin George, Sivasankara Rao Ede, Zhiping Luo
This is a test
Compartir libro
- 350 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Fundamentals of Perovskite Oxides
Synthesis, Structure, Properties and Applications
Gibin George, Sivasankara Rao Ede, Zhiping Luo
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
This textbook entitled Fundamentals of Perovskite Oxides: Synthesis, Structure, Properties and Applications summarizes the structure, synthesis routes, and potential applications of perovskite oxide materials. Since these perovskite-type ceramic materials offer opportunities in a wide range of fields of science and engineering, the chapters are broadly organized into four sections of perovskite-type oxide materials and technology.
- Covers recent developments in perovskite oxides
-
- Serves as a quick reference of perovskite oxides information
-
- Describes novel synthesis routes for nanostructured perovskites
-
- Discusses comprehensive details for various crystal structures, synthesis methods, properties, and applications
-
- Applies to academic education, scientific research, and industrial R&D for materials research in real-world applications like bioengineering, catalysis, energy conversion, energy storage, environmental engineering, and data storage and sensing
-
This book serves as a handy and practical guideline suitable for students, engineers, and researchers working with advanced ceramic materials.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Fundamentals of Perovskite Oxides un PDF/ePUB en línea?
Sí, puedes acceder a Fundamentals of Perovskite Oxides de Gibin George, Sivasankara Rao Ede, Zhiping Luo en formato PDF o ePUB, así como a otros libros populares de Physical Sciences y Clinical Chemistry. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
1 Introduction to Perovskites
Perovskites have been an important class of materials, exhibiting unusual promising functionalities in various transport and physical properties over other traditional ceramic or composite materials. They have received extensive attention more recently in the fields of materials science, physics, chemistry, geology, and engineering. In this chapter, we give a brief introduction to perovskites regarding their history, formation, and classification.
1.1 History of Perovskites
The term perovskite originated from the mineral perovskite (CaTiO3) named after the Russian mineralogist Mr. Lev Alekseyevich von Perovski (1792–1856) (Liu et al. 2017). A photo of the CaTiO3 mineral is shown in Figure 1.1. Nowadays, perovskites represent a wide class of materials with similar or derived crystal structure as that of CaTiO3 with a general formula ABX3, as shown in Figure 1.2. In the structure of perovskites, A and B are cations and X is the anion, and A cations are larger than B cations (Figure 1.2a). Six X anions form an octahedron covering the smaller cation B (Figure 1.2b). Therefore, this perovskite lattice is formed by such apex-connected octahedra with A cations between them (Figure 1.2c).

FIGURE 1.1 A photo of CaTiO3 mineral. (From https://en.wikipedia.org/wiki/Perovskite.)
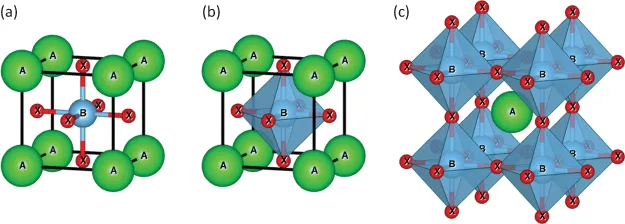
FIGURE 1.2 Perovskite ABX3 lattice. (a) Large A and small B cations, with X anions; (b) octahedron by X anions; (c) octahedra lattice.
Often A-site ion provides the structural integrity to the perovskite structure, and B-site ion determines the properties. CaTiO3 crystallizes into an orthorhombic structure; however, the ideal perovskite structure belongs to the cubic space group (No. 221). Apart from CaTiO3, many naturally existing minerals adopt a perovskite structure, for instance, ilmenite (FeTiO3), MgSiO3, etc. The silicate perovskites containing Mg, Ca, and Fe are assumed as the most abundant solid phase in the earth’s lower mantle at 670–2,900 km from the surface. Moreover, MgSiO3 is stable above 23 GPa (Zhang et al. 2014), and the same is believed to constitute the big planets other than Mars (Umemoto et al. 2006). Many perovskite materials exhibit exceptional properties such as high absorption coefficient, long-range ambipolar charge transport, low exciton-binding energy, high dielectric constant, and ferroelectric properties.
BaTiO3 is the first manmade perovskite synthesized during World War II in 1941; however, it’s naturally existing counterpart benitoite is an extremely rare mineral. BaTiO3 was used as a ferroelectric and piezoelectric material for a wide range of applications (Eshita et al. 2014). Its application as a piezoelectric material is later replaced by lead zirconate titanate Pb(Zr, Ti)O3, another important perovskite. Similarly, SrTiO3 is the first insulator, and the first oxide reported as superconductive below 0.35 K. Even though SrTiO3 was synthesized in the 1950s, its natural counterpart was discovered in 1982. SrTiO3’s first-ever use was as a simulant for diamond due to its resemblance to a diamond, but the softness and high cost resulted in their replacement with other simulants such as yttrium aluminum garnet (YAG), gadolinium gallium garnet (GGG), and cubic zirconia.
1.2 Formation of Perovskites
Since the discovery of BaTiO3, a large number of perovskites with general formula ABO3 have been synthesized and studied for their unique properties pertaining to the presence of two different cations in their lattice. In general, the new perovskite structured materials possess a wide range of properties such as optical, magnetic, thermoelectric, piezoelectric, photochemical, thermochromic, electrochromic, and electrochemical, which are not observed in their ancestors. Besides the general physical and chemical properties, these materials are extensively studied for their applicability as electrode materials for energy storage and scavenging, e.g., LaMnO3, Ba0.5Sr0.5Co0.8Fe0.2O3, etc. Despite the low-cost and ea...