
eBook - ePub
Fundamentals of Perovskite Oxides
Synthesis, Structure, Properties and Applications
Gibin George, Sivasankara Rao Ede, Zhiping Luo
This is a test
Condividi libro
- 350 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Fundamentals of Perovskite Oxides
Synthesis, Structure, Properties and Applications
Gibin George, Sivasankara Rao Ede, Zhiping Luo
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
This textbook entitled Fundamentals of Perovskite Oxides: Synthesis, Structure, Properties and Applications summarizes the structure, synthesis routes, and potential applications of perovskite oxide materials. Since these perovskite-type ceramic materials offer opportunities in a wide range of fields of science and engineering, the chapters are broadly organized into four sections of perovskite-type oxide materials and technology.
-
- Covers recent developments in perovskite oxides
-
- Serves as a quick reference of perovskite oxides information
-
- Describes novel synthesis routes for nanostructured perovskites
-
- Discusses comprehensive details for various crystal structures, synthesis methods, properties, and applications
-
- Applies to academic education, scientific research, and industrial R&D for materials research in real-world applications like bioengineering, catalysis, energy conversion, energy storage, environmental engineering, and data storage and sensing
This book serves as a handy and practical guideline suitable for students, engineers, and researchers working with advanced ceramic materials.
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Fundamentals of Perovskite Oxides è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Fundamentals of Perovskite Oxides di Gibin George, Sivasankara Rao Ede, Zhiping Luo in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Physical Sciences e Clinical Chemistry. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
1 Introduction to Perovskites
Perovskites have been an important class of materials, exhibiting unusual promising functionalities in various transport and physical properties over other traditional ceramic or composite materials. They have received extensive attention more recently in the fields of materials science, physics, chemistry, geology, and engineering. In this chapter, we give a brief introduction to perovskites regarding their history, formation, and classification.
1.1 History of Perovskites
The term perovskite originated from the mineral perovskite (CaTiO3) named after the Russian mineralogist Mr. Lev Alekseyevich von Perovski (1792–1856) (Liu et al. 2017). A photo of the CaTiO3 mineral is shown in Figure 1.1. Nowadays, perovskites represent a wide class of materials with similar or derived crystal structure as that of CaTiO3 with a general formula ABX3, as shown in Figure 1.2. In the structure of perovskites, A and B are cations and X is the anion, and A cations are larger than B cations (Figure 1.2a). Six X anions form an octahedron covering the smaller cation B (Figure 1.2b). Therefore, this perovskite lattice is formed by such apex-connected octahedra with A cations between them (Figure 1.2c).

FIGURE 1.1 A photo of CaTiO3 mineral. (From https://en.wikipedia.org/wiki/Perovskite.)
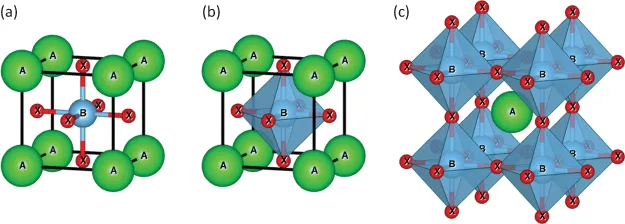
FIGURE 1.2 Perovskite ABX3 lattice. (a) Large A and small B cations, with X anions; (b) octahedron by X anions; (c) octahedra lattice.
Often A-site ion provides the structural integrity to the perovskite structure, and B-site ion determines the properties. CaTiO3 crystallizes into an orthorhombic structure; however, the ideal perovskite structure belongs to the cubic space group (No. 221). Apart from CaTiO3, many naturally existing minerals adopt a perovskite structure, for instance, ilmenite (FeTiO3), MgSiO3, etc. The silicate perovskites containing Mg, Ca, and Fe are assumed as the most abundant solid phase in the earth’s lower mantle at 670–2,900 km from the surface. Moreover, MgSiO3 is stable above 23 GPa (Zhang et al. 2014), and the same is believed to constitute the big planets other than Mars (Umemoto et al. 2006). Many perovskite materials exhibit exceptional properties such as high absorption coefficient, long-range ambipolar charge transport, low exciton-binding energy, high dielectric constant, and ferroelectric properties.
BaTiO3 is the first manmade perovskite synthesized during World War II in 1941; however, it’s naturally existing counterpart benitoite is an extremely rare mineral. BaTiO3 was used as a ferroelectric and piezoelectric material for a wide range of applications (Eshita et al. 2014). Its application as a piezoelectric material is later replaced by lead zirconate titanate Pb(Zr, Ti)O3, another important perovskite. Similarly, SrTiO3 is the first insulator, and the first oxide reported as superconductive below 0.35 K. Even though SrTiO3 was synthesized in the 1950s, its natural counterpart was discovered in 1982. SrTiO3’s first-ever use was as a simulant for diamond due to its resemblance to a diamond, but the softness and high cost resulted in their replacement with other simulants such as yttrium aluminum garnet (YAG), gadolinium gallium garnet (GGG), and cubic zirconia.
1.2 Formation of Perovskites
Since the discovery of BaTiO3, a large number of perovskites with general formula ABO3 have been synthesized and studied for their unique properties pertaining to the presence of two different cations in their lattice. In general, the new perovskite structured materials possess a wide range of properties such as optical, magnetic, thermoelectric, piezoelectric, photochemical, thermochromic, electrochromic, and electrochemical, which are not observed in their ancestors. Besides the general physical and chemical properties, these materials are extensively studied for their applicability as electrode materials for energy storage and scavenging, e.g., LaMnO3, Ba0.5Sr0.5Co0.8Fe0.2O3, etc. Despite the low-cost and ea...