
eBook - ePub
Alkaloids
A Treasury of Poisons and Medicines
Shinji Funayama, Geoffrey A. Cordell
This is a test
Compartir libro
- 294 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Alkaloids
A Treasury of Poisons and Medicines
Shinji Funayama, Geoffrey A. Cordell
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Alkaloids are a large group of structurally complex natural products displaying a wide range of biological activities. The purpose of Alkaloids: A Treasury of Poisons and Medicines is to classify, for the first time, the alkaloids isolated from the natural sources until now. The book classifies all of the alkaloids by their biosynthetic origins. Of interest to the organic chemistry and medicinal chemistry communities involved in drug discovery and development, this book describes many alkaloids isolated from the medicinal plants, including those used in Japanese Kampo medicine.
- Classifies and lists alkaloids from natural sources
- Occurrence and biosynthetic pathways of alkaloids
- Indicates key uses and bioactivity of alkaloids
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Alkaloids un PDF/ePUB en línea?
Sí, puedes acceder a Alkaloids de Shinji Funayama, Geoffrey A. Cordell en formato PDF o ePUB, así como a otros libros populares de Sciences physiques y Chimie organique. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
Categoría
Sciences physiquesCategoría
Chimie organiqueChapter 1
Alkaloids Derived from Phenylalanine and Tyrosine
Abstract
This chapter introduces those alkaloids derived from phenylalanine and tyrosine.
Keywords
Aristolochic acid; Berberine; Chelidonine; Coclaurine; Colchicine; d-tubocurarine; Dopamine; Emetine; Galanthamine; L-DOPA; Lycorine; Morphine; Phenylethylamine; Thyroxine
The thousands of alkaloids derived from phenylalanine and tyrosine possess a wide range of important biological activities, and several of them are pharmaceutical agents, present in various traditional medicines in various systems, or serve as biological tools. In this chapter, those alkaloids are discussed, from the simplest alkaloids to those that represent more complex chemical structures. The coclaurine-type alkaloids are one of the simplest of these alkaloids, and are described in Section 1.4. Among them, reticuline, in its antipodal forms, is an important biosynthetic precursor of various alkaloids, including such alkaloids as berberine and morphine. Some of the alkaloids of this type are derived through highly complicated, and incompletely understood, biosynthetic pathways. For example, it is very difficult to elucidate the original amino acid derivations of colchicine (Section 1.11) and lycorine (Section 1.13) from a superficial examination of their chemical structures.
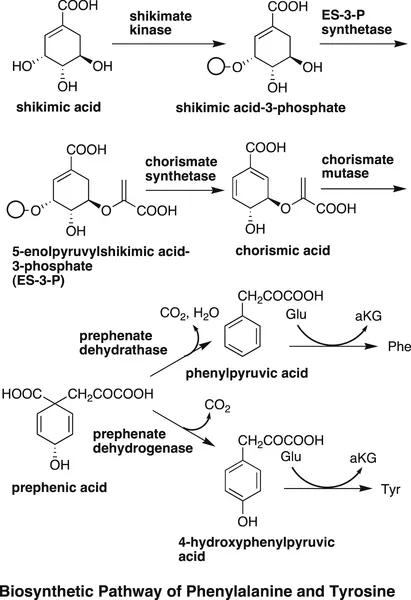
Both phenylalanine and tyrosine are derived from chorismic acid, which is itself derived from shikimic acid-3-phosphate through the shikimic acid pathway. In this sequence, chorismic acid is first transformed into prephenic acid by chorismate mutase. If prephenic acid is converted into phenylpyruvic acid by the action of prephenate dehydratase and a transaminase, phenylalanine is formed. On the other hand, if it is transformed into 4-hydroxyphenylpyruvic acid by the action of prephenate dehydrogenase, followed by a transaminase reaction, then tyrosine is formed.
1.1
Phenylethylamines (Phenethylamines)
Peyote (Lophophora williamsii, syn. Anhalonium williamisii) is a cactus and member of the family Cactaceae, and grows wild in the deserts of Mexico and the southern United States [1]. The cactus is also cultivated in Japan as a decorative plant and known as “Ubatama.”
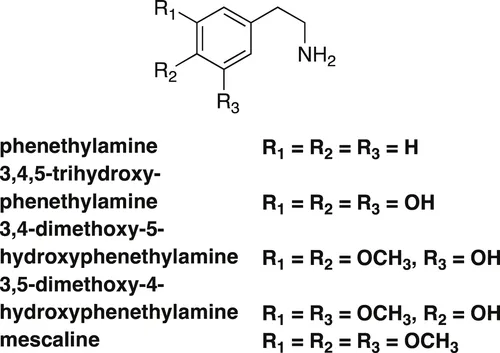
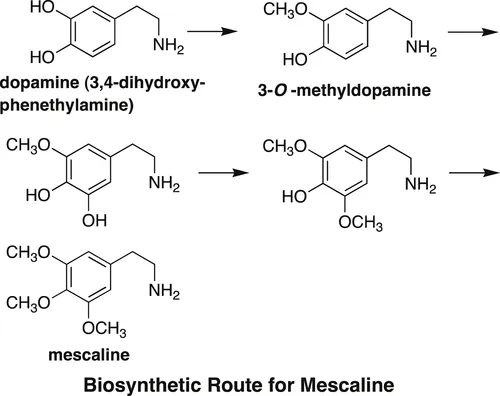
This cactus is an important source of the so-called phenylethylamine (phenethylamine) alkaloids with a C6C2N skeleton. The main component of the alkaloid mixture is mescaline. The name mescaline is derived from the name of the cactus, which is also known as “mescal buttons.” Mescaline is known to possess hallucinatory effects and a number of undesirable side effects. With respect to the biosynthesis of mescaline, it was shown that L-tyrosine is oxidized to give L-DOPA (L-3,4-dihydroxyphenylalanine), which is transformed to the biosynthetic precursor dopamine (3,4-dihydroxyphenethylamine) [2], which is selectively O-methylated to afford 3-O-methyldopamine, a key biosynthetic precursor of the alkaloid. The direct biosynthetic precursor of mescaline was determined to be 3,5-dimethoxy-4-hydroxyphenethylamine, because 3,4,5-trihydroxy phenethylamine and 3,4-dimethoxy-5-hydroxyphenethylamine were not incorporated into the biosynthetic pathway to mescaline [2].
Hordenine and N-methyltyramine are isolates from the young roots of Hordeum vulgare var. hexastichon (Poaceae), and are simple phenylethylamine-type alkaloids. The biosynthetic precursor of these alkaloids is considered to be tyramine, derived from tyrosine. dl-[2-14C]-Tyrosine was fed to H. vulgare var. hexastichon 4 days after germination, and hordenine and N-methyltyramine were isolated after 11 days from the roots. Both alkaloids possessed 14C label at the α-carbon. It was also found that dl-[2-14C]-tyrosine was more effectively incorporated into N-methyltyramine than into hordenine, and no tyramine was detected in the extract. So, the incorporated tyrosine was converted into tyramine and methylated immediately to give N-methyltyramine. Subsequent steps form hordenine by the methylation of N-methyltyramine [3].
On the other hand, it was clarified by the incorporation of labeled methionine that the methyl groups incorporated during the biosynthesis of N-methyltyramine and hordenine were derived from methionine. The yield of N-methyltyramine in these experiments is less than half that of hordenine; however, the incorporation of 14C into N-methyltyramine is 1.5 times that of hordenine. This indicates that N-methyltyramine is not formed by the demethylation of hordenine, but th...