
eBook - ePub
Alkaloids
A Treasury of Poisons and Medicines
Shinji Funayama,Geoffrey A. Cordell
This is a test
Buch teilen
- 294 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Alkaloids
A Treasury of Poisons and Medicines
Shinji Funayama,Geoffrey A. Cordell
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Alkaloids are a large group of structurally complex natural products displaying a wide range of biological activities. The purpose of Alkaloids: A Treasury of Poisons and Medicines is to classify, for the first time, the alkaloids isolated from the natural sources until now. The book classifies all of the alkaloids by their biosynthetic origins. Of interest to the organic chemistry and medicinal chemistry communities involved in drug discovery and development, this book describes many alkaloids isolated from the medicinal plants, including those used in Japanese Kampo medicine.
- Classifies and lists alkaloids from natural sources
- Occurrence and biosynthetic pathways of alkaloids
- Indicates key uses and bioactivity of alkaloids
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Alkaloids als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Alkaloids von Shinji Funayama,Geoffrey A. Cordell im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Naturwissenschaften & Organische Chemie. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Thema
NaturwissenschaftenThema
Organische ChemieChapter 1
Alkaloids Derived from Phenylalanine and Tyrosine
Abstract
This chapter introduces those alkaloids derived from phenylalanine and tyrosine.
Keywords
Aristolochic acid; Berberine; Chelidonine; Coclaurine; Colchicine; d-tubocurarine; Dopamine; Emetine; Galanthamine; L-DOPA; Lycorine; Morphine; Phenylethylamine; Thyroxine
The thousands of alkaloids derived from phenylalanine and tyrosine possess a wide range of important biological activities, and several of them are pharmaceutical agents, present in various traditional medicines in various systems, or serve as biological tools. In this chapter, those alkaloids are discussed, from the simplest alkaloids to those that represent more complex chemical structures. The coclaurine-type alkaloids are one of the simplest of these alkaloids, and are described in Section 1.4. Among them, reticuline, in its antipodal forms, is an important biosynthetic precursor of various alkaloids, including such alkaloids as berberine and morphine. Some of the alkaloids of this type are derived through highly complicated, and incompletely understood, biosynthetic pathways. For example, it is very difficult to elucidate the original amino acid derivations of colchicine (Section 1.11) and lycorine (Section 1.13) from a superficial examination of their chemical structures.
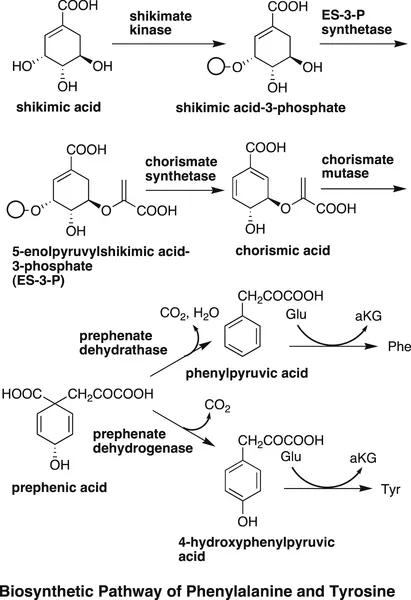
Both phenylalanine and tyrosine are derived from chorismic acid, which is itself derived from shikimic acid-3-phosphate through the shikimic acid pathway. In this sequence, chorismic acid is first transformed into prephenic acid by chorismate mutase. If prephenic acid is converted into phenylpyruvic acid by the action of prephenate dehydratase and a transaminase, phenylalanine is formed. On the other hand, if it is transformed into 4-hydroxyphenylpyruvic acid by the action of prephenate dehydrogenase, followed by a transaminase reaction, then tyrosine is formed.
1.1
Phenylethylamines (Phenethylamines)
Peyote (Lophophora williamsii, syn. Anhalonium williamisii) is a cactus and member of the family Cactaceae, and grows wild in the deserts of Mexico and the southern United States [1]. The cactus is also cultivated in Japan as a decorative plant and known as “Ubatama.”
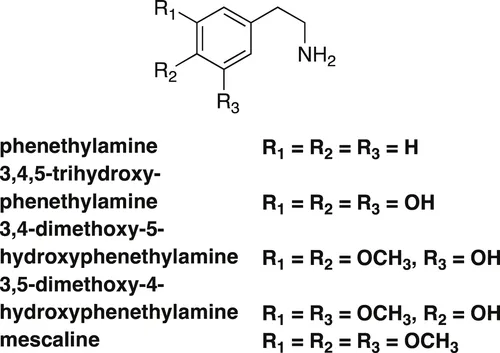
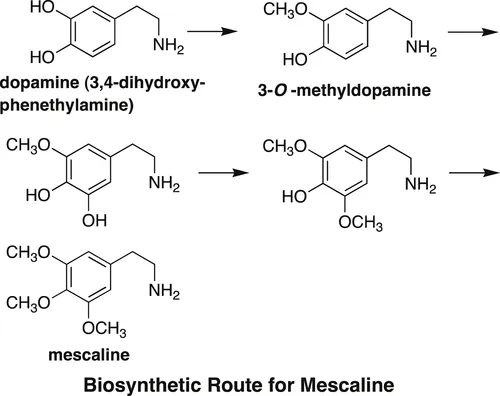
This cactus is an important source of the so-called phenylethylamine (phenethylamine) alkaloids with a C6C2N skeleton. The main component of the alkaloid mixture is mescaline. The name mescaline is derived from the name of the cactus, which is also known as “mescal buttons.” Mescaline is known to possess hallucinatory effects and a number of undesirable side effects. With respect to the biosynthesis of mescaline, it was shown that L-tyrosine is oxidized to give L-DOPA (L-3,4-dihydroxyphenylalanine), which is transformed to the biosynthetic precursor dopamine (3,4-dihydroxyphenethylamine) [2], which is selectively O-methylated to afford 3-O-methyldopamine, a key biosynthetic precursor of the alkaloid. The direct biosynthetic precursor of mescaline was determined to be 3,5-dimethoxy-4-hydroxyphenethylamine, because 3,4,5-trihydroxy phenethylamine and 3,4-dimethoxy-5-hydroxyphenethylamine were not incorporated into the biosynthetic pathway to mescaline [2].
Hordenine and N-methyltyramine are isolates from the young roots of Hordeum vulgare var. hexastichon (Poaceae), and are simple phenylethylamine-type alkaloids. The biosynthetic precursor of these alkaloids is considered to be tyramine, derived from tyrosine. dl-[2-14C]-Tyrosine was fed to H. vulgare var. hexastichon 4 days after germination, and hordenine and N-methyltyramine were isolated after 11 days from the roots. Both alkaloids possessed 14C label at the α-carbon. It was also found that dl-[2-14C]-tyrosine was more effectively incorporated into N-methyltyramine than into hordenine, and no tyramine was detected in the extract. So, the incorporated tyrosine was converted into tyramine and methylated immediately to give N-methyltyramine. Subsequent steps form hordenine by the methylation of N-methyltyramine [3].
On the other hand, it was clarified by the incorporation of labeled methionine that the methyl groups incorporated during the biosynthesis of N-methyltyramine and hordenine were derived from methionine. The yield of N-methyltyramine in these experiments is less than half that of hordenine; however, the incorporation of 14C into N-methyltyramine is 1.5 times that of hordenine. This indicates that N-methyltyramine is not formed by the demethylation of hordenine, but th...