
eBook - ePub
Sustainable and Functional Redox Chemistry
Shinsuke Inagi, Shinsuke Inagi
This is a test
Compartir libro
- 386 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Sustainable and Functional Redox Chemistry
Shinsuke Inagi, Shinsuke Inagi
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Mimicking nature's efficiency and sustainability in organic chemistry is a major goal for future chemists; redox reactions are a key element in a variety of fields ranging from synthesis and catalysis to materials chemistry and analytical applications. Sustainability is increasingly becoming a consideration in synthesis and functional chemistry and an essential element for the next generation of chemistry in academia and industry. This book represents a compilation of the latest advancements in functional redox chemistry and demonstrates its importance in achieving a more sustainable future. This book is an ideal companion for any postgraduate students or researchers interested in sustainability in academia and industry.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Sustainable and Functional Redox Chemistry un PDF/ePUB en línea?
Sí, puedes acceder a Sustainable and Functional Redox Chemistry de Shinsuke Inagi, Shinsuke Inagi en formato PDF o ePUB, así como a otros libros populares de Physical Sciences y Chemistry. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
Sustainable Redox Reaction
Chapter 1
Redox-mediated Electrochemical Cyclization Reactions
a State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China Email: [email protected]
1.1 Introduction
More than 90% of common organic compounds contain rings.1,2 As a result, the search for efficient means for the construction of cyclic structures has been constantly pursued in the field of organic synthesis.
Organic electrochemistry employs electric current to drive organic synthetic reactions and is attracting renewed interest in the past decade.3,4 Since electrons do not produce reagent-related waste and are among the cheapest reagents for chemical synthesis, organic electrochemistry holds great promise in developing green and sustainable synthetic methods.5 While the majority of the reported organic electrosynthetic reactions rely on direct electrolysis, indirect electrolysis with mediators has been increasingly explored, especially in the past few years.6–9 The use of mediators not only allows the reactions to proceed under mild electrode potentials to reduce energy consumption and increase selectivity10,11 but also significantly expands the scope of organic electrosynthesis to many redox inactive compounds through atom transfer catalysis12 or C–H bond activation.13–16 In addition, electrolysis with a redox mediator allows the generation of radical intermediates in the bulk solution away from the electrode surface to avoid electrode passivation and reduce their local concentration.9 Key to the success of indirect electrosynthesis is the development of mediators that can function under electrochemical conditions. Efforts in the past few years have significantly expanded the list of mediators, some of which are listed in Scheme 1.1A, leading to the rapid development of indirect electrosynthesis. The mediators promote electrochemical reactions through an outer-sphere or inner-sphere mechanism (Scheme 1.1B). In the latter case, a transient adduct is formed between the mediator and substrate either after or before electron transfer on the electrode. In this context, many redox-mediated electrochemical cyclization reactions have been disclosed for the synthesis of various hetero- and carbocycles and will be the focus of this chapter (Scheme 1.1C). These reactions proceed mainly through radical or ionic cyclization to forge the ring structures.
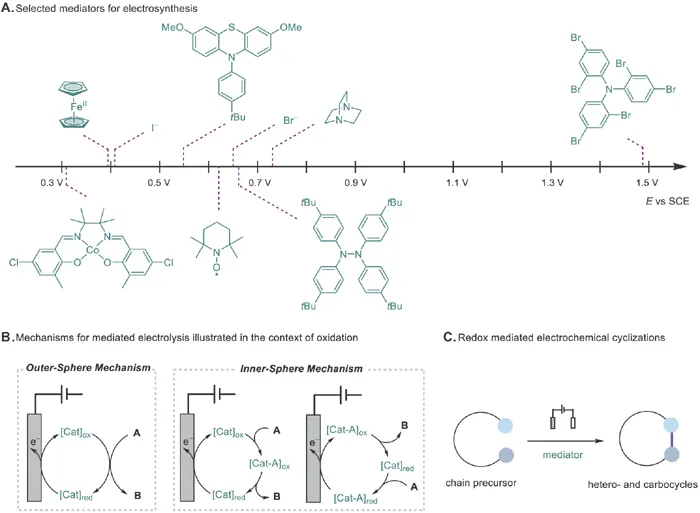
Scheme 1.1 Redox-mediated electrochemical cyclization reactions.
1.2 Radical Cyclization Reactions
Radical cyclization reactions are effective for the synthesis of ring structures because of the versatile reactivity of radical species and the possibility for cyclization cascades.17,18 In this context, several redox strategies have been developed for the electrochemical generation of various heteroatom- and carbon-centered radical species. These reactive species react to form several classes of hetero- and carbocycles by cyclization onto the tethered π-systems, 1,5-hydrogen atom transfer, or intermolecular addition to alkenes or alkynes to induce cyclizations.
1.2.1 Cyclization Reactions of Heteroatom-centered Radicals
Nitrogen-centered radicals (NCRs) are attractive intermediates for the construction of C–N bonds.19–22 These reactive species are commonly produced through the cleavage of a weak N–heteroatom bond.23 The current trend is to generate NCRs from stable and easily available N–H precursors.24–28
The Xu group reported in 2014 an early example of redox-mediated electrochemical generation of NCRs from anilides using 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) as the mediator (Scheme 1.2A).29 Mechanistically, the anilide 1d is deprotonated by hydroxide generated at the cathode and then oxidized to amidyl radical 3via single electron transfer (SET) by anodically generated oxoammonium salt (TEMPO+). Intermediate 3 undergoes 5-exo-trig cyclizations to give carbon-centered radical 4, which is trapped with TEMPO to generate the final aminooxygenation product 2d. A similar aminooxygenation reaction was later achieved using a continuous flow electrochemical microreactor by Wirth and coworkers.30 Although the use of TEMPO as the mediator limits the reaction to aminooxygenation,31 this work proves that redox catalysis can be an effective strategy in developing electrochemically driven radical reactions. Importantly, the electrode potentials needed for these mediated electrochemical reactions are much lower than those for the anilide substrates. In addition, the continuous generation of the requisite base at the cathode to promote the oxidation reaction obviates the need to add stoichiometric strong bases and avoids base-induced side reactions.
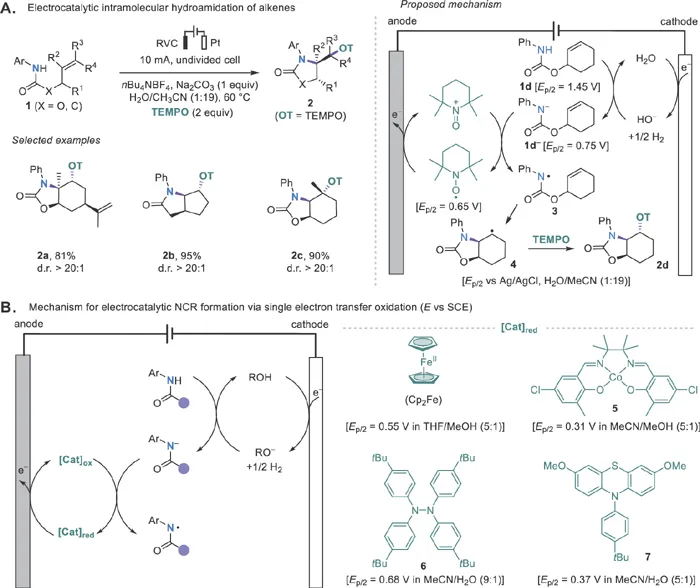
Scheme 1.2 Mediated electrochemical generation of NCRs.
The Xu group then went on to develop several new catalytic systems for the oxidative generation of NCRs from anilides employing ferrocene (Cp2Fe), cobalt salen complex 5, tetraarylhydrazine 6, or phenothiazine 7 as the catalysts (Scheme 1.2B).26 All these mediators catalyze the anilide oxidation through outer-sphere electron transfer except for 5, which oxidizes the anilides through inner-sphere electron transfer.32 These electrophilic NCRs generated under the electrocatalytic conditions undergo monocyclization or cyclization cascades onto tethered alkenes (Scheme 1.3A–C)33–35 or alkynes (Scheme 1.3D–E)36–38 to form several types of useful N-heterocycles. Note that ferrocene is not a good catalyst for anilide oxidation in aqueous solutions because of its reduced stability and oxidation potential in these solvents. As a result, organic mediators such as 7 or 6 are used for the cyclizations of 12 and 24, respectively.34,38 The use of redox catalysis allows the formation of heterocyclic products that are oxidized at lower potentials than the starting anilides. Direct electrolysis can also be employed to promote NCR formation and cyclizations but often requires an increase in oxidation potential from substrate to product.39–42 Otherwise, further oxidation of the product can occur.43
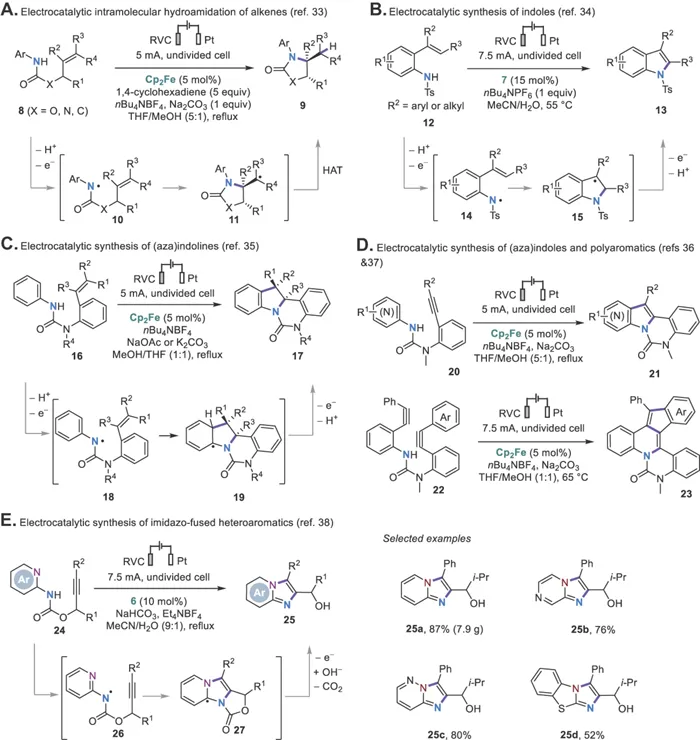
Scheme 1.3 Electrocatalytic cyclization reactions via NCRs.
Aza-Wacker-type cyclization reac...