
eBook - ePub
Sustainable and Functional Redox Chemistry
Shinsuke Inagi, Shinsuke Inagi
This is a test
Partager le livre
- 386 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Sustainable and Functional Redox Chemistry
Shinsuke Inagi, Shinsuke Inagi
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Mimicking nature's efficiency and sustainability in organic chemistry is a major goal for future chemists; redox reactions are a key element in a variety of fields ranging from synthesis and catalysis to materials chemistry and analytical applications. Sustainability is increasingly becoming a consideration in synthesis and functional chemistry and an essential element for the next generation of chemistry in academia and industry. This book represents a compilation of the latest advancements in functional redox chemistry and demonstrates its importance in achieving a more sustainable future. This book is an ideal companion for any postgraduate students or researchers interested in sustainability in academia and industry.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Sustainable and Functional Redox Chemistry est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Sustainable and Functional Redox Chemistry par Shinsuke Inagi, Shinsuke Inagi en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Physical Sciences et Chemistry. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Sustainable Redox Reaction
Chapter 1
Redox-mediated Electrochemical Cyclization Reactions
a State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China Email: [email protected]
1.1 Introduction
More than 90% of common organic compounds contain rings.1,2 As a result, the search for efficient means for the construction of cyclic structures has been constantly pursued in the field of organic synthesis.
Organic electrochemistry employs electric current to drive organic synthetic reactions and is attracting renewed interest in the past decade.3,4 Since electrons do not produce reagent-related waste and are among the cheapest reagents for chemical synthesis, organic electrochemistry holds great promise in developing green and sustainable synthetic methods.5 While the majority of the reported organic electrosynthetic reactions rely on direct electrolysis, indirect electrolysis with mediators has been increasingly explored, especially in the past few years.6–9 The use of mediators not only allows the reactions to proceed under mild electrode potentials to reduce energy consumption and increase selectivity10,11 but also significantly expands the scope of organic electrosynthesis to many redox inactive compounds through atom transfer catalysis12 or C–H bond activation.13–16 In addition, electrolysis with a redox mediator allows the generation of radical intermediates in the bulk solution away from the electrode surface to avoid electrode passivation and reduce their local concentration.9 Key to the success of indirect electrosynthesis is the development of mediators that can function under electrochemical conditions. Efforts in the past few years have significantly expanded the list of mediators, some of which are listed in Scheme 1.1A, leading to the rapid development of indirect electrosynthesis. The mediators promote electrochemical reactions through an outer-sphere or inner-sphere mechanism (Scheme 1.1B). In the latter case, a transient adduct is formed between the mediator and substrate either after or before electron transfer on the electrode. In this context, many redox-mediated electrochemical cyclization reactions have been disclosed for the synthesis of various hetero- and carbocycles and will be the focus of this chapter (Scheme 1.1C). These reactions proceed mainly through radical or ionic cyclization to forge the ring structures.
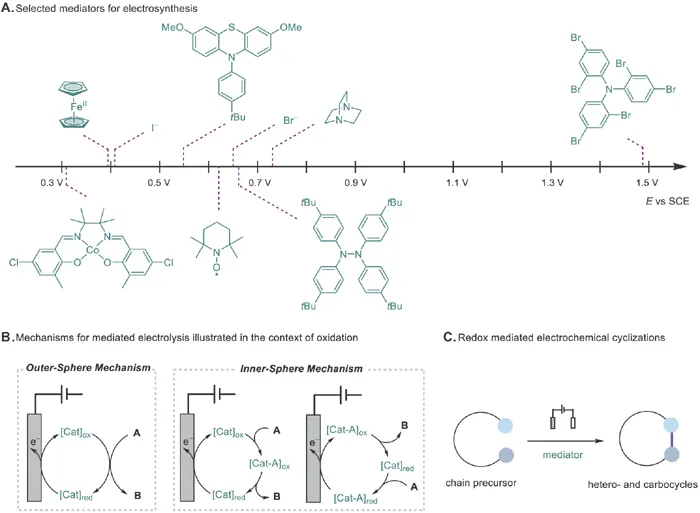
Scheme 1.1 Redox-mediated electrochemical cyclization reactions.
1.2 Radical Cyclization Reactions
Radical cyclization reactions are effective for the synthesis of ring structures because of the versatile reactivity of radical species and the possibility for cyclization cascades.17,18 In this context, several redox strategies have been developed for the electrochemical generation of various heteroatom- and carbon-centered radical species. These reactive species react to form several classes of hetero- and carbocycles by cyclization onto the tethered π-systems, 1,5-hydrogen atom transfer, or intermolecular addition to alkenes or alkynes to induce cyclizations.
1.2.1 Cyclization Reactions of Heteroatom-centered Radicals
Nitrogen-centered radicals (NCRs) are attractive intermediates for the construction of C–N bonds.19–22 These reactive species are commonly produced through the cleavage of a weak N–heteroatom bond.23 The current trend is to generate NCRs from stable and easily available N–H precursors.24–28
The Xu group reported in 2014 an early example of redox-mediated electrochemical generation of NCRs from anilides using 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) as the mediator (Scheme 1.2A).29 Mechanistically, the anilide 1d is deprotonated by hydroxide generated at the cathode and then oxidized to amidyl radical 3via single electron transfer (SET) by anodically generated oxoammonium salt (TEMPO+). Intermediate 3 undergoes 5-exo-trig cyclizations to give carbon-centered radical 4, which is trapped with TEMPO to generate the final aminooxygenation product 2d. A similar aminooxygenation reaction was later achieved using a continuous flow electrochemical microreactor by Wirth and coworkers.30 Although the use of TEMPO as the mediator limits the reaction to aminooxygenation,31 this work proves that redox catalysis can be an effective strategy in developing electrochemically driven radical reactions. Importantly, the electrode potentials needed for these mediated electrochemical reactions are much lower than those for the anilide substrates. In addition, the continuous generation of the requisite base at the cathode to promote the oxidation reaction obviates the need to add stoichiometric strong bases and avoids base-induced side reactions.
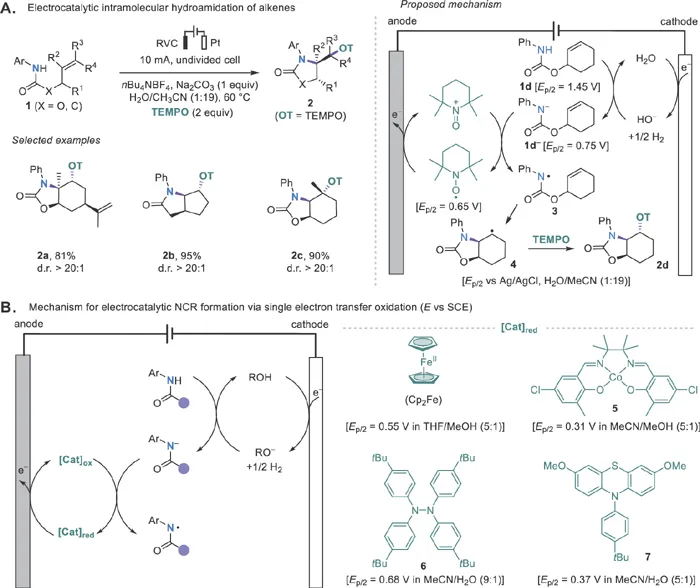
Scheme 1.2 Mediated electrochemical generation of NCRs.
The Xu group then went on to develop several new catalytic systems for the oxidative generation of NCRs from anilides employing ferrocene (Cp2Fe), cobalt salen complex 5, tetraarylhydrazine 6, or phenothiazine 7 as the catalysts (Scheme 1.2B).26 All these mediators catalyze the anilide oxidation through outer-sphere electron transfer except for 5, which oxidizes the anilides through inner-sphere electron transfer.32 These electrophilic NCRs generated under the electrocatalytic conditions undergo monocyclization or cyclization cascades onto tethered alkenes (Scheme 1.3A–C)33–35 or alkynes (Scheme 1.3D–E)36–38 to form several types of useful N-heterocycles. Note that ferrocene is not a good catalyst for anilide oxidation in aqueous solutions because of its reduced stability and oxidation potential in these solvents. As a result, organic mediators such as 7 or 6 are used for the cyclizations of 12 and 24, respectively.34,38 The use of redox catalysis allows the formation of heterocyclic products that are oxidized at lower potentials than the starting anilides. Direct electrolysis can also be employed to promote NCR formation and cyclizations but often requires an increase in oxidation potential from substrate to product.39–42 Otherwise, further oxidation of the product can occur.43
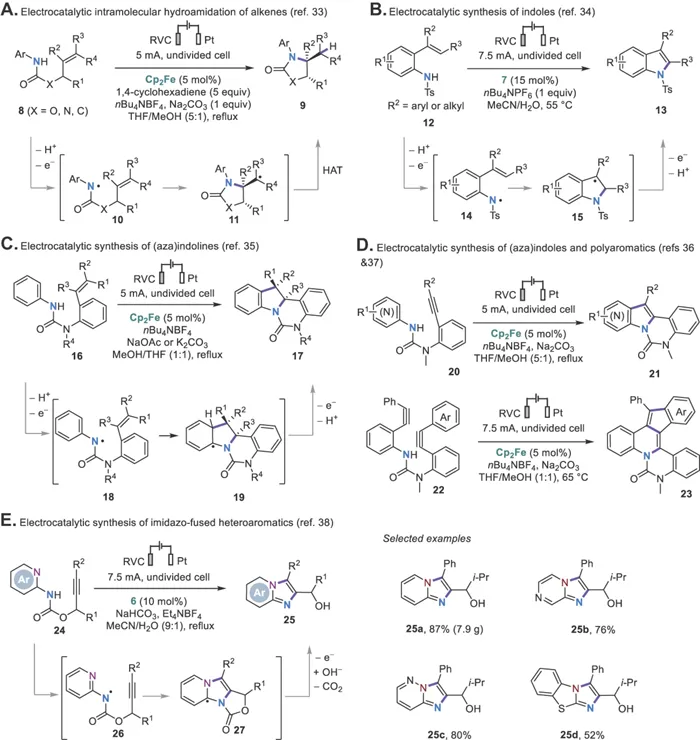
Scheme 1.3 Electrocatalytic cyclization reactions via NCRs.
Aza-Wacker-type cyclization reac...