
eBook - ePub
Introduction to Statistical Mechanics
Solutions to Problems
John Dirk Walecka
This is a test
Compartir libro
- 244 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Introduction to Statistical Mechanics
Solutions to Problems
John Dirk Walecka
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Statistical mechanics is concerned with defining the thermodynamic properties of a macroscopic sample in terms of the properties of the microscopic systems of which it is composed. The previous book Introduction to Statistical Mechanics provided
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Introduction to Statistical Mechanics un PDF/ePUB en línea?
Sí, puedes acceder a Introduction to Statistical Mechanics de John Dirk Walecka en formato PDF o ePUB, así como a otros libros populares de Naturwissenschaften y Mathematische & Computerphysik. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
Categoría
NaturwissenschaftenCategoría
Mathematische & ComputerphysikChapter 1
Introduction
Problem 1.1 Prove from Eq. (1.1) that the integral in Eq. (1.3) is independent of path.
Solution to Problem 1.1
Equation (1.1) states the following integral around a closed cycle vanishes
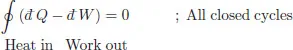
Pick two points on the cycle (A, B) with specified thermodynamic variables. Let C1 denote that part of the cycle running from A → B, and C2 that part of the cycle returning from B → A. Then the above states
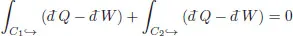
where the arrows indicate the direction during the cycle. Since both the heat flow and work are algebraic and change sign if the change occurs in the opposite direction, the integral changes sign if the trajectory is traversed in the opposite direction1
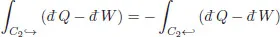
Hence the previous relation can be re-written as
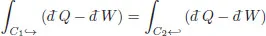
This states that the integral is the same whether we go from A to B along C1 or along C2 in the opposite direction. Since the cycle containing the points (A, B) is arbitrary, the integral
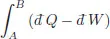
is independent of the path from A → B.
Problem 1.2 Start from either statement of the second law, and see how far you can get in verifying the statements leading to Eqs. (1.5) and (1.6); then compare with [Zemansky (1968)].
Solution to Problem 1.2
The two equivalent statements of the second law of thermodynamics are given at the start of section 1.1.2:
(1)Kelvin: It is impossible to construct an engine that, operating in a cycle, will produce no effect other that extraction of heat from a reservoir and performance of an equivalent amount of work.
(2)Clausius: It is impossible to construct a device that, operating in a cycle, will produce no effect other than the transfer of heat from a cooler to a hotter body.
We shall not provide a rigorous derivation of the consequences, but leave that to a basic course in thermodynamics.2 The key element is to establish that all reversible engines operating between two heat baths at distinct temperatures T1 > T2 have the same efficiency, equal to that of a Carnot engine, from which Eqs. (1.5)–(1.7) follow. The argument goes something like this. Consider two such engines operating between the two heat baths, and let the second operate in the reverse direction. Suppose the first absorbs heat Q1 at T1, produces work W, and expels heat Q2 at T2. Now put the heat Q2 and work W into the second engine. If it does not expel exactly the same heat Q1 into the bath at T1, then the combined engines constitute a device that can be arranged to violate the second statement of the second law.
Problem 1.3 (a) Why does each point on the dotted curve in Fig. 1.2 in the text correspond to a given T?3
(b) How could one carry out the Carnot cycles shown in Fig. 1.2 in the text in a continuous manner with each segment being covered only once?
(c) Why is it unnecessary for the construction of the entropy to actually traverse opposing segments of the adiabats in (b)?
(d) Show that the total heat input and total work output in (b) satisfy Q = W.
Solution to Problem 1.3
(a) A perfect gas obeys the equation of state PV = nRT (see, for example, Prob. 1.4). Thus if we specify (P, V), we also specify T;
(b) If we simply take the assembly around the outer segments of all the Carnot cycles, omitting all the common segments traversed in opposite directions, we move around the loop in a clockwise direction, in a continuous manner, with each segment being covered only once;
(c) The opposing segments along the adiabats in Fig. 1.2 in the text are traversed in a reversible manner, and along each of them the reversible heat flow vanishes, dQR = 0. Hence there is no change in entropy along those segments;
(d) Since the gas retu...