
eBook - ePub
Introduction to Statistical Mechanics
Solutions to Problems
John Dirk Walecka
This is a test
Partager le livre
- 244 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Introduction to Statistical Mechanics
Solutions to Problems
John Dirk Walecka
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Statistical mechanics is concerned with defining the thermodynamic properties of a macroscopic sample in terms of the properties of the microscopic systems of which it is composed. The previous book Introduction to Statistical Mechanics provided
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Introduction to Statistical Mechanics est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Introduction to Statistical Mechanics par John Dirk Walecka en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Naturwissenschaften et Mathematische & Computerphysik. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Sujet
NaturwissenschaftenSous-sujet
Mathematische & ComputerphysikChapter 1
Introduction
Problem 1.1 Prove from Eq. (1.1) that the integral in Eq. (1.3) is independent of path.
Solution to Problem 1.1
Equation (1.1) states the following integral around a closed cycle vanishes
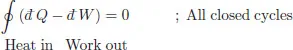
Pick two points on the cycle (A, B) with specified thermodynamic variables. Let C1 denote that part of the cycle running from A → B, and C2 that part of the cycle returning from B → A. Then the above states
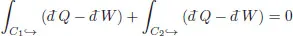
where the arrows indicate the direction during the cycle. Since both the heat flow and work are algebraic and change sign if the change occurs in the opposite direction, the integral changes sign if the trajectory is traversed in the opposite direction1
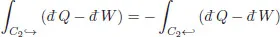
Hence the previous relation can be re-written as
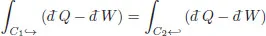
This states that the integral is the same whether we go from A to B along C1 or along C2 in the opposite direction. Since the cycle containing the points (A, B) is arbitrary, the integral
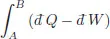
is independent of the path from A → B.
Problem 1.2 Start from either statement of the second law, and see how far you can get in verifying the statements leading to Eqs. (1.5) and (1.6); then compare with [Zemansky (1968)].
Solution to Problem 1.2
The two equivalent statements of the second law of thermodynamics are given at the start of section 1.1.2:
(1)Kelvin: It is impossible to construct an engine that, operating in a cycle, will produce no effect other that extraction of heat from a reservoir and performance of an equivalent amount of work.
(2)Clausius: It is impossible to construct a device that, operating in a cycle, will produce no effect other than the transfer of heat from a cooler to a hotter body.
We shall not provide a rigorous derivation of the consequences, but leave that to a basic course in thermodynamics.2 The key element is to establish that all reversible engines operating between two heat baths at distinct temperatures T1 > T2 have the same efficiency, equal to that of a Carnot engine, from which Eqs. (1.5)–(1.7) follow. The argument goes something like this. Consider two such engines operating between the two heat baths, and let the second operate in the reverse direction. Suppose the first absorbs heat Q1 at T1, produces work W, and expels heat Q2 at T2. Now put the heat Q2 and work W into the second engine. If it does not expel exactly the same heat Q1 into the bath at T1, then the combined engines constitute a device that can be arranged to violate the second statement of the second law.
Problem 1.3 (a) Why does each point on the dotted curve in Fig. 1.2 in the text correspond to a given T?3
(b) How could one carry out the Carnot cycles shown in Fig. 1.2 in the text in a continuous manner with each segment being covered only once?
(c) Why is it unnecessary for the construction of the entropy to actually traverse opposing segments of the adiabats in (b)?
(d) Show that the total heat input and total work output in (b) satisfy Q = W.
Solution to Problem 1.3
(a) A perfect gas obeys the equation of state PV = nRT (see, for example, Prob. 1.4). Thus if we specify (P, V), we also specify T;
(b) If we simply take the assembly around the outer segments of all the Carnot cycles, omitting all the common segments traversed in opposite directions, we move around the loop in a clockwise direction, in a continuous manner, with each segment being covered only once;
(c) The opposing segments along the adiabats in Fig. 1.2 in the text are traversed in a reversible manner, and along each of them the reversible heat flow vanishes, dQR = 0. Hence there is no change in entropy along those segments;
(d) Since the gas retu...