
eBook - ePub
MRI in Practice
Catherine Westbrook, John Talbot
This is a test
Compartir libro
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
MRI in Practice
Catherine Westbrook, John Talbot
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
MRI in Practice continues to be the number one reference book and study guide for the registry review examination for MRI offered by the American Registry for Radiologic Technologists (ARRT). This latest edition offers in-depth chapters covering all core areas, including: basic principles, image weighting and contrast, spin and gradient echo pulse sequences, spatial encoding, k-space, protocol optimization, artefacts, instrumentation, and MRI safety.
- The leading MRI reference book and study guide.
- Now with a greater focus on the physics behind MRI.
- Offers, for the first time, equations and their explanations and scan tips.
- Brand new chapters on MRI equipment, vascular imaging and safety.
- Presented in full color, with additional illustrations and high-quality MRI images to aid understanding.
- Includes refined, updated and expanded content throughout, along with more learning tips and practical applications.
- Features a new glossary.
MRI in Practice is an important text for radiographers, technologists, radiology residents, radiologists, and other students and professionals working within imaging, including medical physicists and nurses.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es MRI in Practice un PDF/ePUB en línea?
Sí, puedes acceder a MRI in Practice de Catherine Westbrook, John Talbot en formato PDF o ePUB, así como a otros libros populares de Medicina y Tecnología y suministros médicos. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
1
Basic principles
- Introduction
- Atomic structure
- Motion in the atom
- MR-active nuclei
- The hydrogen nucleus
- Alignment
- Net magnetic vector (NMV)
- Precession and precessional (Larmor) frequency
- Precessional phase
- Resonance
- MR signal
- The free induction decay (FID) signal
- Pulse timing parameters
After reading this chapter, you will be able to:
- Describe the structure of the atom.
- Explain the mechanisms of alignment and precession.
- Understand the concept of resonance and signal generation.
INTRODUCTION
The basic principles of magnetic resonance imaging (MRI) form the foundation for further understanding of this complex subject. It is important to grasp these ideas before moving on to more complicated topics in this book.
There are essentially two ways of explaining the fundamentals of MRI: classically and via quantum mechanics. Classical theory (accredited to Sir Isaac Newton and often called Newtonian theory) provides a mechanical view of how the universe (and therefore how MRI) works. Using classical theory, MRI is explained using the concepts of mass, spin, and angular momentum on a large or bulk scale. Quantum theory (accredited to several individuals including Max Planck, Albert Einstein, and Paul Dirac) operates at a much smaller, subatomic scale and refers to the energy levels of protons, neutrons, and electrons. Although classical theory is often used to describe physical principles on a large scale and quantum theory on a subatomic level, there is evidence that all physical principles are explained using quantum concepts [1]. However, for our purposes, this chapter mainly relies on classical perspectives because they are generally easier to understand. Quantum theory is only used to provide more detail when required.
In this chapter, we explore the properties of atoms and their interactions with magnetic fields as well as the mechanisms of excitation and relaxation.
ATOMIC STRUCTURE
All things are made of atoms. Atoms are organized into molecules, which are two or more atoms arranged together. The most abundant atom in the human body is hydrogen, but there are other elements such as oxygen, carbon, and nitrogen. Hydrogen is most commonly found in molecules of water (where two hydrogen atoms are arranged with one oxygen atom; H2O) and fat (where hydrogen atoms are arranged with carbon and oxygen atoms; the number of each depends on the type of fat).
The atom consists of a central nucleus and orbiting electrons (Figure 1.1). The nucleus is very small, one millionth of a billionth of the total volume of an atom, but it contains all the atom’s mass. This mass comes mainly from particles called nucleons, which are subdivided into protons and neutrons. Atoms are characterized in two ways.
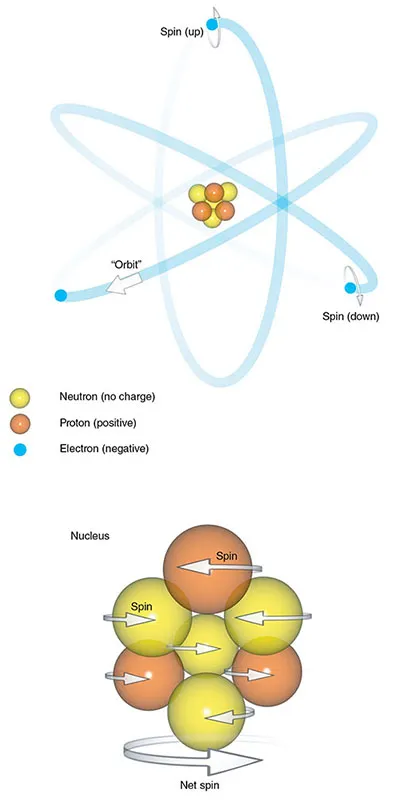
Figure 1.1 The atom.
- The atomic number is the sum of the protons in the nucleus. This number gives an atom its chemical identity.
- The mass number or atomic weight is the sum of the protons and neutrons in the nucleus.
The number of neutrons and protons in a nucleus is usually balanced so that the mass number is an even number. In some atoms, however, there are slightly more or fewer neutrons than protons. Atoms of elements with the same number of protons but a different number of neutrons are called isotopes.
Electrons are particles that spin around the nucleus. Traditionally, this is thought of as analogous to planets orbiting around the...