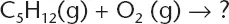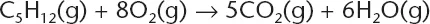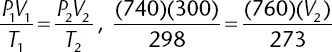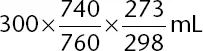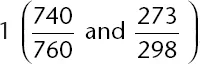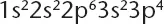![]()
PART I
INTRODUCTION
![]()
Chapter 1
Keys for Success on the AP Chemistry Exam
The textbooks that are typically used in college-level general chemistry courses contain thousands of facts and concepts packed into (at least) several hundred pages. If the AP Chemistry exam tested all of that information, earning a good score on the exam would be a daunting one.
Studying for the AP Chemistry exam requires you to be a pragmatic learner who can delineate the important (tested) material from the material that is “interesting to know.” This book will help you to become more focused in your study, and help you to streamline your efforts. Your chances of scoring well on the exam will be enhanced by pinpointing what is absolutely necessary and ignoring the “fluff.” The keys to success include the following:
1. The Content and Format of the AP Chemistry Exam
The Advanced Placement Chemistry curriculum is based on the content of an introductory chemistry course taught at the college level. The topics taught in the class reflect major topics that are presented in a number of college-level textbooks. The curriculum of the course also includes some inquiry-based laboratory situations, application of what the College Board calls scientific practices and chemical calculations.
In order to succeed on the exam, students need to master the basic concepts of chemistry and apply these concepts to various situations in a traditional test format. The content of the exam is based on the following six “big ideas”:
1. The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements of atoms. These atoms retain their identity in chemical reactions. (See Part II: Atoms and Elements.)
2. Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, or molecules and the forces between them. (See Part III: Bonding.)
3. Changes in matter involve the rearrangement and/or reorganization of atoms and/or the transfer of electrons. (See Part IV: Chemical Reactions.)
4. Rates of chemical reactions are determined by details of the molecular collisions. (See Part V: Rates of Reaction.)
5. The laws of thermodynamics describe the essential role of energy and explain and predict the direction of changes in matter. (See Part VI: Chemical Thermodynamics.)
6. Any bond or intermolecular attraction that can be formed can be broken. These two processes are in a dynamic competition, sensitive to initial conditions and external perturbations. (See Part VII: Equilibrium.)
The AP Chemistry exam is 3 hours in length and consists of a 90-minute multiple-choice question section and a 90-minute free-response question section. There are 60 multiple-choice questions and 7 free-response questions.
Section I. 60 multiple-choice questions (90 minutes) – 50% of the grade
In this section of the exam, NO calculator is allowed, but access to the periodic table and an equations and constants sheet is allowed.
Section II. 7 free-response questions (3 “long” questions and 4 “short” questions, with multiple parts) (90 minutes) – 50% of the grade In this section of the exam, a calculator is allowed along with access to the periodic table and an equations and constants sheet.
2. The Multiple-Choice Questions
Multiple-choice questions test the knowledge, understanding, and application of the “big ideas” and science practices in the AP Chemistry course. For example:
When the chemical equation below is completed and balanced, what statement best describes the reaction?
(A) 1 mole of pentane is consumed and 6 moles of water are produced.
(B) 1 mole of pentane is produced and 6 moles of water are consumed.
(C) 1 mole of pentane is consumed and 1 mole of water is produced.
(D) 1 mole of pentane is produced and 1 mole of water is consumed.
The correct answer is (A). The equation represents a combustion reaction that will produce carbon dioxide and water. When balanced the equation is:
It is essential to know these basic ideas when confronted by this type of question. This book will give you the content that you need to know to successfully answer the questions.
In addition, calculation-based chemistry questions are also part of this section. However, since no calculator is allowed, some multiple-choice questions will ask you to select the correct mathematical setup, or require you to do some simple arithmetic or estimation. For example:
At 25°C, 300 milliliters of an ideal gas exerts a pressure of 740 mm Hg. What is the volume of the gas under conditions of 0°C and 760 mm Hg?
(A) 2.68 mL
(B) 26.8 mL
(C) 268 mL
(D) 2680 mL
The correct answer is (C). Using the combined gas law formula, remembering to convert temperatures to Kelvin and plugging in values, the following mathematical setup is determined:
Solving for V
2 gives
. Although you have no calculator, you should see that 300 mL is being multiplied by two numbers that are slightly less than
, and, as such, the answer must be slightly less than 300 mL; 268 mL is the correct answer choice. Estimation should allow you to find the correct answer without the need for a calculator (i.e., without the need to find the exact answer).
Multiple-Choice Question Formats
There are two types of multiple-choice questions that make up the majority of Section I of the AP Chemistry exam, with Type II making up a maximum of 50% of the exam.
Type I. Traditional multiple-choice questions with answer choices (A) through (D). There is only one correct answer. For example:
Atoms of element X have the electron configuration shown below.
The compound most ...