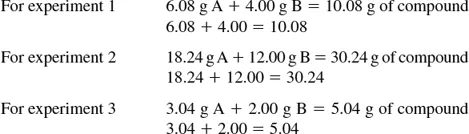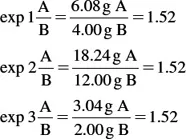![]()
CHAPTER 1
Introduction
1.1 Matter and Its Properties
1.1.1 Definition of Matter
Matter occupies space and possesses mass. Mass is an intrinsic property of matter.
Weight is the force, due to gravity, with which an object is attracted to the earth.
Force and mass are related to each other by Newton’s equation (Newton’s Law), F = ma, where F = force, m = mass, and a = acceleration. Weight and mass are related by the equation w = mg, where w = weight, m = mass, and g = acceleration due to gravity.
Note that the terms “mass” and “weight” are often (incorrectly) used interchangeably in most literature.
1.1.2 States of Matter
Matter occurs in three states or phases: solid, liquid, and gas. A solid has both a definite size and shape. A liquid has a definite volume but takes the shape of the container, and a gas has neither definite shape nor definite volume.
1.1.3 Composition of Matter
Matter is divided into two categories: distinct substances and mixtures. Distinct substances are either elements or compounds. An element is made up of only one kind of atom. A compound is composed of two or more kinds of atoms joined together in a definite composition.
Mixtures contain two or more distinct substances more or less intimately jumbled together. A mixture has no unique set of properties: it possesses the properties of the substances of which it is composed.
In a homogeneous mixture, the composition and physical properties are uniform throughout. Only a single phase is present. A homogeneous mixture can be gaseous, liquid, or solid. A heterogeneous mixture, such as oil and water, is not uniform and consists of two or more phases.
1.1.4 Properties of Matter
Extensive properties, such as mass and volume, depend on the size of the sample. Intensive properties, such as melting point, boiling point, and density, are independent of sample size.
Physical properties of matter are those properties that can be observed, usually with our senses. Examples of physical properties are physical state, color, and melting point.
Chemical properties of a substance are observed only in chemical reactions involving that substance.
Reactivity is a chemical property that refers to the tendency of a substance to undergo a particular chemical reaction.
Chemical changes are those that involve the breaking and/or forming of chemical bonds, as in a chemical reaction.
Physical changes do not result in the formation of new substances. Changes in state are physical changes.
1.2 Conservation of Matter
1.2.1 Law of Conservation of Matter
In a chemical change, matter is neither created nor destroyed, but only changed from one form to another. This law requires that “material balance” be maintained in chemical equations.
1.3 Laws of Definite and Multiple Proportions
1.3.1 Law of Definite Proportions
A pure compound is always composed of the same elements combined in a definite proportion by mass.
Problem Solving Example:
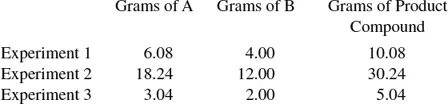
It has been determined experimentally that two elements, A and B, react chemically to produce a compound or compounds. Experimental data obtained on combining proportions of the elements are:
(a) Which two laws of chemical change are illustrated by the above data? (b) If 80 g of element B combines with 355 g of a third element C, what weight of A will combine with 71 g of element C? (c) If element B is oxygen, what is the equivalent weight of element C?
(a) If one adds the weight of A to the weight of B and obtains the weight of the compound formed, the law of conservation of matter is illustrated. This law states that there is no detectable gain or loss of matter in a chemical change. Using the data from the experiments described, you find the following:
From these calculations one can see that the law of conservation of matter is shown.
Another important law of chemistry is the law of definite proportions. This law is stated: when elements combine to form a given compound, they do so in a fixed and invariable ratio by mass. One can check to see if this law is adhered to by calculating the ratio of the weight of A to the weight of B in the three experiments. If all of these ratios are equal, the law of definite proportions is shown.
The law of definite proportions is illustrated here.
(b) From the law of definite proportions, one can find the number of grams of B that will combine with 71 g of ...