
eBook - ePub
Male Infertility in Reproductive Medicine
Diagnosis and Management
Botros Rizk, Ashok Agarwal, Edmund Sabanegh Jr., Botros Rizk, Ashok Agarwal, Edmund S. Sabanegh Jr.
This is a test
Partager le livre
- 205 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Male Infertility in Reproductive Medicine
Diagnosis and Management
Botros Rizk, Ashok Agarwal, Edmund Sabanegh Jr., Botros Rizk, Ashok Agarwal, Edmund S. Sabanegh Jr.
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
This useful illustrated text summarizes for an audience of clinicians in Reproductive Medicine the practical essentials of what they need to know about diagnosis and management of the infertile male patient, whether they need to instruct or liaise with a colleague or undertake the procedures themselves.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Male Infertility in Reproductive Medicine est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Male Infertility in Reproductive Medicine par Botros Rizk, Ashok Agarwal, Edmund Sabanegh Jr., Botros Rizk, Ashok Agarwal, Edmund S. Sabanegh Jr. en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Medicina et Teoria, pratica e riferimenti medici. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
1
The Pathophysiology of Male Infertility
Pallav Sengupta and Chak-Lam Cho
KEY POINTS
• Pathophysiology of male infertility involves complex multivariate mechanisms.
• Dysregulations of hormonal axes and endocrine cross-talks adversely affect male reproductive functions.
• Testicular disruptions directly impair semen parameters.
• Posttesticular impairments afflict spermatozoal maturation and transport.
Introduction
The male reproductive system apparently possesses simplistic functions so as to produce sperm and testosterone, but the underlying mechanisms are far more complex and yet to be completely revealed. Such elusive mechanisms of male reproductive functions have led to poor understanding of the actual causatives of male infertility in about 50% of the cases [1]. Disruption of male fertility may be reflected by impaired sperm parameters through multivariate factors at different levels [2,3,4]. Etiologies of male infertility may act at the pretesticular or neuroendocrine regulatory levels. Other factors may directly affect intratesticular sites, thereby afflicting the functions of Sertoli cells, Leydig cells, and germ cells. Disruptions can also occur at the posttesticular strata, impairing sperm maturation and transport. Besides the conventional concept of pathophysiology of male infertility, there is advent in male reproductive immunology as well as reproductive genetics and epigenetics, modulations of which may induce varying forms of impairment to the male fecundity. Proper evaluation of male infertility at different levels is essential for its effective management. Targeted treatment to specific male factor with or without assisted reproductive techniques (ART) may be adopted for management of male infertility [5].
This chapter is a concise synopsis of the pathophysiology of male infertility merging the classical and modern postulations. It summarizes the concepts of male reproductive functions and their regulatory factors. Finally the mechanisms by which impairment of the reproductive functions or their regulators, individually or in concerts, leading to male infertility are illustrated.
Male Reproductive Physiology: An Overview
Pristine perception of both morphology and physiology of male reproductive system facilitates conceptualization of the complex pathophysiological mechanisms of male infertility. The male reproductive system has three fundamental functions: Production of spermatozoa (spermatogenesis) and hormones (steroidogenesis), as well as storage followed by ejaculation of the sperm into the female reproductive tract [6]. However, accomplishment of these functions require orchestrated action of the testicular cells including the germ cells, Sertoli cells, and Leydig cells in response to the endocrine regulation. The male reproductive system along with its regulatory entity comprises of brain centers, which regulate pituitary release of gonadotropins and sexual behavior; a pair of testes, which produce sperm and hormones; a ductal system (vas deferens and epididymis), which stores and transports sperm; accessory sex glands (seminal vesicles, prostate, and bulbourethral glands) to support sperm viability; and the penis [7].
Spermatogenesis and steroidogenesis are under endocrine regulation via the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [8]. The hypothalamus is known to be the center of information processing as per external and internal cues. Via the pulsatile release of gonadotropin-releasing hormone (GnRH), it stimulates the secretion by anterior pituitary, LH and FSH, which binds to receptors on the Leydig cells, and Sertoli cells, respectively. Leydig cells reside within the interstitial compartments and produce testosterone. Sertoli cells lie along the lining of the seminiferous tubules, supporting the germ cells to develop through the stages of spermatogenesis. Sertoli cells have receptors for both FSH and testosterone and produce estradiol at low levels. Another contribution of the Sertoli cells is the productions of glycoprotein hormones (inhibin, activin, and follistatin) that modulate FSH secretion [9]. Testosterone is the main androgen that sends feedback to the hypothalamus and pituitary, regulates spermatogenesis directly, monitors sexual behavior, and serves as the primary male sex hormone that aids primary and secondary sex development.
The duct system, comprising of epididymis, vas deferens, and urethra, stores the sperm until they acquire the capability to fertilize with sufficient motility and then transports them to the female genital tract through the penis [6].
Alterations in one or more of the components of the reproductive system are accompanied by modulations in other reproductive organs and their endocrine regulations.
Etiologies of Male Infertility
The clinical definition of “infertility” itself not only often poses a conceptual enigma that both subfertile and infertile couples are put under the same category but also refers to failure in attaining pregnancy within the first year of unprotected intercourse. However, many subfertile couples actually may not conceive within this stipulated time and are included under the definition of being infertile [10]. In addition, among all the cases of male infertility, about 60%–75% are idiopathic and remain undiagnosed [11]. Diagnosis of male infertility probably covers a number of different etiologies, which again is a mechanistic paradox and several hypotheses attempt to explain the multivariate causes of the same. The physiological disruptions resulting in male infertility may be related to failure in sperm production, impaired sperm morphology and functions, problems in transmission along the duct system through the penis during ejaculation, secretory disturbances of the accessory glands, and endocrine imbalances. There lies an array of concepts to justify these events individually or in combinations and most of the time, the exact mechanism is difficult to specify. In many cases, male infertility remains just a mystery. The contributing factors ranges from severe to moderate pathological conditions, systemic causes, environmental factors, lifestyle factors, and metabolic distress to oxidative stress (Figure 1.1). This chapter aims to address the perplexity unveiling the physiological mechanisms paving the way to male infertility, explaining every strata of male reproductive functions at the pretesticular, testicular, and posttesticular levels.
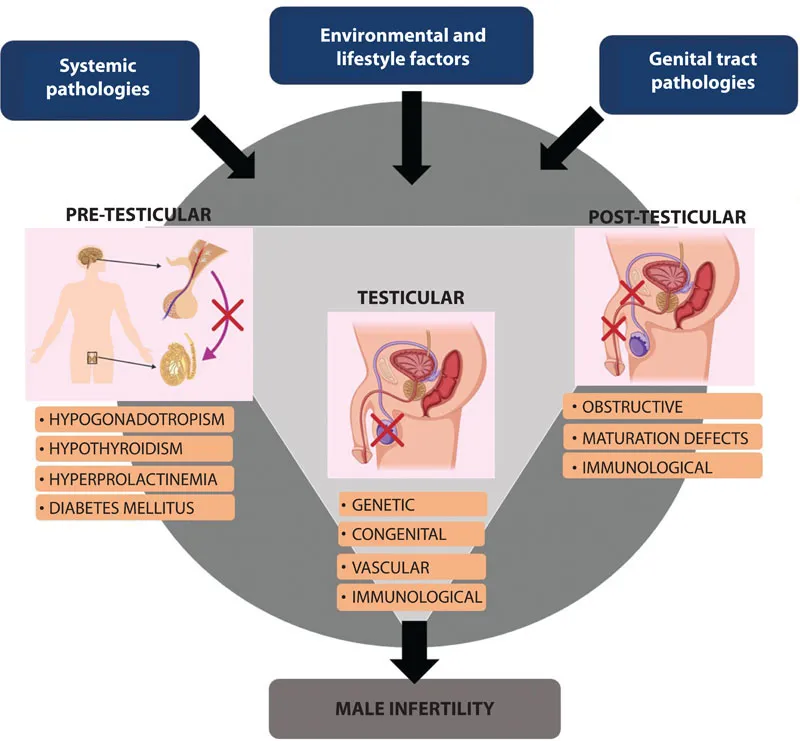
Pretesticular Pathophysiology
Immaculate coordination of the hypothalamic-pituitary-testicular axis with other related hormones determine functioning of the male reproductive system. The hypothalamus via its pulsatile secretion of GnRH stimulates the pituitary gonadotropins, LH, and FSH, which regulate testicular steroidogenesis and spermatogenesis. Inhibin and activin from the testes in turn operate feedback mechanisms that influence the secretion of both hypothalamic GnRH and subsequent pituitary gonadotropins [12].
Research since the last decade, procures that besides the pivotal classical scheme of the hypothalamic-pituitary-gonadal (HPG) axis, there are several other components playing vital roles in the regulation of male reproductive functions. Among these are the groups of small RFamide peptides consisting of the motif Arg-Phe-NH2 at C-terminus, namely, gonadotropin-inhibiting hormone (GnIH) and its related peptides [8]. Another essential 54-amino-acid peptide, Kisspeptin, encoded by the KiSS-1 gene, has been identified. This peptide, which activates the G protein–coupled receptor (GPR54) in the hypothalamus, is reportedly a major trigger for puberty and can supposedly even kindle precocious puberty in men [13].
HPG axis may be disoriented by the influence of an inestimable number of internal and external cues, the most common being via the stress hormones, several adipokines, and the opioid system. A disrupted HPG axis results in inadequate sex steroids and inhibin production and in turn, loss of negative feedback to regulate hypothalamus and pituitary secretions. Consequently, there is an increase in serum activin and unregulated release of GnRH and gonadotropins [14]. This undesired elevation of LH and FSH culminates in male reproductive dysfunctions [15]. Hence, dysregulation of the HPG axis actually modulates concentrations of gonadal hormones and alters the sensitivity of their respective hippocampal receptors, resulting in disoriented hormone-receptor signaling and abnormal elevation in neuronal GnRH, LH, and activin signaling [16]. Stress may also induce elevated levels of reactive oxygen species (ROS), which may trigger oxidative stress (OS). This may lead to lipid peroxidation (LPO) in Leydig cells and germ cells, disrupt lipoproteins, fragment proteins, and inhibit steroidogenic enzyme activities [17]. OS mediates its detrimental effects on male fertility by reduction in testosterone production by affecting Leydig cells or indirectly via disruptions in endocrine regulations of hypothalamus or anterior pituitary [18].
Interruption or ceased GnRH release and subsequent inhibition of LH and FSH secretions lead to hypogonadotropic hypogonadism (HH). Secondary and tertiary HH owing to hypothalamic and pituitary hormones deficiencies, respectively, are different from primary or testicular dependent factors. Secondary or tertiary HH is characterized by normal or low gonadotropin levels with low testosterone concentration [12]. Congenital abnormalities resulting from GnRH deficiency can either occur singly (normosmic congenital HH) or along with hyposmia or anosmia, which is called Kallmann syndrome. Besides testosterone insufficiency, fertility problems, and anosmia, patients with Kallmann syndrome often experience other neurologic and cardiac disorders. Hypogonadism is thus a threat to male fecundity and systemic functions and can be caused by various factors including aging [19], obesity [20], and type 2 diabetes mellitus [21]. Steroidogenesis gradually declines with aging and reports suggest men older than 60 years general...