
eBook - ePub
Case Studies in Infection Control
Meera Chand, John Holton
This is a test
Partager le livre
- 300 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Case Studies in Infection Control
Meera Chand, John Holton
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Case Studies in Infection Control has 25 cases, each focusing on an infectious disease, which illustrate the critical aspects of infection control and prevention. Scenarios in the cases are real events from both community and hospital situations, and written by experts. Although brief comments are included in relation to the organism, diagnosis, and treatment the main emphasis is on the case, its epidemiology, and how the situation should be managed from the perspective of infection control and prevention. Each case also has multiple choice questions and answers as well as listing international guidelines and references. All the cases will be an invaluable learning tool for anyone studying or practicing infection control.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Case Studies in Infection Control est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Case Studies in Infection Control par Meera Chand, John Holton en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Medicine et Internal Medicine & Diagnosis. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
ANTHRAX | CASE 1 |
1SpR, Rare and Imported Pathogens Laboratory, Public Health England and Hospital for Tropical Diseases, University College London Hospitals NHS Foundation Trust, London, UK.
2Head and Clinical Services Director, Rare and Imported Pathogens Laboratory, Public Health England, UK.
This case occurred during an outbreak of anthrax in the UK, which caused a series of serious soft tissue infections among injecting drug users. Skin and soft tissue infections are common in injecting drug users, about 35% of whom are estimated annually of developing a significant infection. There have been significant outbreaks in UK drug users resulting from contamination of street drugs with spore-forming organisms, the most notable being an outbreak of Clostridium novii in 2000, but also C. tetani (tetanus) and a variety of Bacillus species over the years.
In this case, a 45-year-old woman had a severe soft tissue infection surrounding a groin injection site, which involved the perineum and left buttock. When she arrived at the hospital, she was grossly septic with tissue necrosis and massive oedema surrounding the injection site. Anthrax was suspected early because there were concurrent cases of anthrax in injecting drug users in other UK centres.
INVESTIGATION OF THE CASE
Tissue samples were tested in a specialist Containment Level 3 laboratory. DNA of Bacillus anthracis was detected by polymerase chain reaction (PCR) of debrided tissue, and this organism was also subsequently cultured on agar. Toxin testing was performed on serum samples. An enzyme immunoassay for B. anthracis Lethal Factor (LF) toxin, a 3-protein exotoxin complex produced by B. anthracis that contributes to its virulence, was positive, and another enzyme-linked immunoassay (EIA) for Protective Antigen (PA) was equivocal.
CLINICAL MANAGEMENT
The principles of clinical management of cases of cutaneous anthrax are the same as those for any severe necrotizing soft tissue infection:
• to prevent toxin formation and organism multiplication (antibiotics)
• to remove the infectious nidus where possible (surgical debridement, usually only in cutaneous disease)
• to neutralize existing toxin (anthrax immune globulin, anti-anthrax toxin monoclonal antibodies, for example, Raxibacumab)
Bacillus anthracis is generally susceptible to penicillin (although resistance has been reported on several occasions), chloramphenicol, tetracycline, erythromycin, streptomycin, fluoroquinolones, and linezolid, but not to cephalosporins or trimethoprim-sulfamethoxazole.
In this case, extensive soft tissue debridement was performed in the operating theatre and high-dose antibiotics (benzylpenicillin 1.2 g every four hours and clindamycin 600 mg every six hours) were commenced. The patient made a good recovery following debridement. Other potential therapies, such as human anthrax immunoglobulin (used to eliminate circulating toxins) and Raxibacumab (a human IgG1 monoclonal antibody directed at the protective antigen of B. anthracis), were considered but not used.
PREVENTION OF FURTHER CASES
Control of anthrax in the hospital setting and waste management
From a public health perspective, the key concern with anthrax is that it is a severe disease with a high mortality, which can contaminate the environment with infectious spores of extreme longevity.
Infection control in the healthcare setting
In general, universal infection control precautions apply. Person-to-person spread of inhalational anthrax has never been documented, and cutaneous anthrax from contact with a human case is extremely rare. This limited transmission is because anthrax bacteria are not themselves capable of establishing infection and spores, by which infection can be transmitted, are only formed in suitable conditions that are usually found outside the human body. Respiratory isolation of cases is therefore not required, although standard personal protective equipment (PPE), including gloves, aprons, and visors (if there is a risk of splash), is advised.
Particular precautions are required in the management of waste, human tissues, and items contaminated with blood and body fluid from anthrax cases because these may be contaminated with bacteria that, once outside the body, form spores and can lead to onward transmission. PPE is necessary when handling such samples. If clothing or sheets are grossly contaminated with blood or body fluids, washing may not remove the remote risk posed by anthrax spores, and accepted advice is to have the clothing incinerated or autoclaved (in standard autoclave conditions - 121°C for 15 minutes under 1.05 kg/cm2 pressure). Minimally contaminated or noncontaminated clothing should be laundered separately from other people’s items in a washing machine at the hottest cycle possible.
Waste management
Segregation of waste into separate management streams at the point of production is vital to good waste management. In general, waste is divided into two streams (hazardous and nonhazardous) based on the threat it poses to the environment and the public. Hazardous waste is further divided into clinical waste and nonclinical waste. Clinical waste may be potentially infectious (that is, contaminated with human blood or tissue) or non-infectious (medicinal waste, cytotoxic waste, amalgam, chemical waste including laboratory, X-ray, and photochemicals, radioactive waste, and gypsum). Waste containers and bags are colour coded according to the type of waste they contain and the ultimate treatment designated for that waste, as described in the UK in Health Technical Memorandum (HTM) 07-01: Safe Management of Healthcare Waste. In the US, each state issues regulations that mandate standards for the management of medical waste, and there is further federal-level legislation implemented by agencies such as the Department of Transport (DOT), the Environmental Protection Agency (EPA), and the Occupational Safety and Health Administration (OSHA).
As an example, practicalities of medical waste disposal in the UK are summarized in Figure 1.1. For ease of use, disposal pathways are colour coded according to the type of waste and the disposal procedures necessary to make it safe. The yellow waste stream is used for waste that is infectious but that has an additional characteristic so that it must be incinerated in a suitably licensed or permitted facility, rather than simply being treated. Recognized examples are waste containing chemicals from human or animal healthcare, or waste contaminated with Advisory Committee of Dangerous Pathogens (ACDP) Category 3 pathogens, such as anthrax. Red stream waste is also incinerated but is limited to anatomical samples.
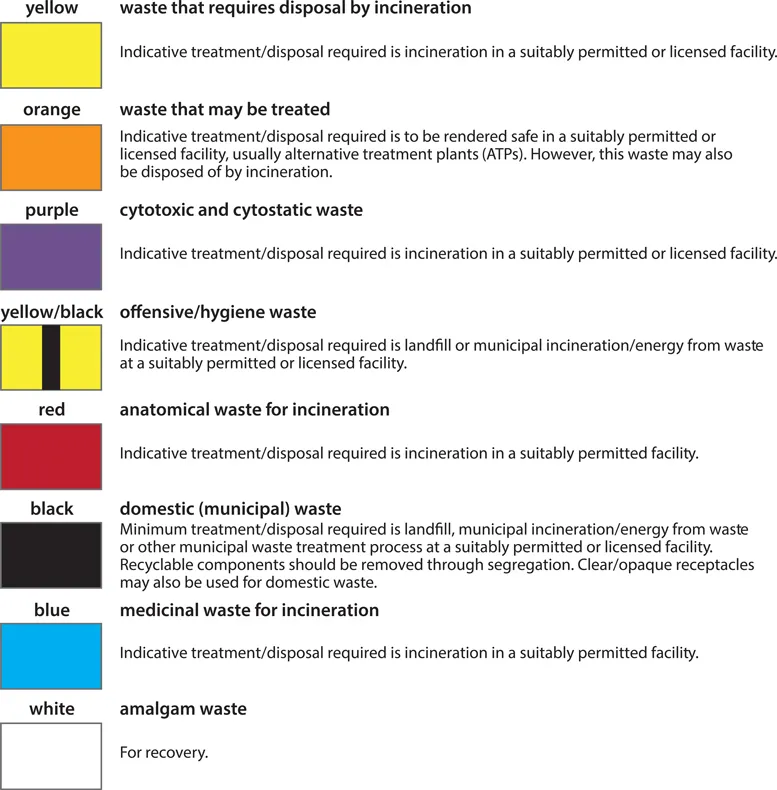
Figure 1.1. Medical waste disposal practicalities in the UK. (Contains public sector information licensed under the Open Government Licence v3.0.)
Orange stream infectious waste may be treated to render it safe prior to final disposal, but is not necessarily incinerated. This waste stream must not contain chemicals, amalgam, medicines, or anatomical waste. In addition, it should not contain waste that is non-infectious (for example, domestic waste) or that has additional characteristics that require incineration (medicinal, chemical, anatomical). Treatment of this waste usually involves maceration, followed by sterilization in a continuous-flow autoclave, known as a hydroclave. The hardiness of anthrax spores means that contaminated waste and tissue pose a unique threat in this waste stream because they can contaminate the macerator prior to sterilization, with attendant exposure risks for operators and maintenance staff. All materials known or suspected of being contaminated with anthrax must therefore be disposed of in the yellow stream, except for debrided human tissues, which are disposed of in the red stream.
Where practicable, medical equipment and mattresses should be decontaminated prior to disposal. Once decontaminated, any infectious risk should be eliminated, although the equipment may still retain hazardous properties that will be subject to statutory waste management controls. If no hazardous properties remain (for example, decontaminated mattresses with the impervious cover intact), the item may be disposed of as domestic waste. Heavily soiled or infectious mattresses should be disposed of as potentially infectious clinical waste.
Unfortunately, some potentially serious lapses of infection control occurred in the management of this case. During surgery, debrided tissue was disposed of incorrectly by being designated as clinical waste to be treated (orange stream), rather than hazardous anatomical waste to be incinerated (red stream). In order to manage the risk from the contaminated waste, a biocontainment team was sent to the waste processing facility. It was fortunate that the waste processing facility was not operating and that a 10-day backlog of waste had accumulated, meaning that no environmental contamination had occurred. Infectious tissue had not leaked into the environment, but the exact location was unclear as orange clinical waste is o...