
eBook - ePub
Nonionic Surfactants
Polyoxyalkylene Block Copolymers
Vaughn Nace
This is a test
Partager le livre
- 284 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Nonionic Surfactants
Polyoxyalkylene Block Copolymers
Vaughn Nace
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Focuses on copolymers made from sequential block polymerizations of ethylene oxide, propylene oxide and 1, 2-butylene oxide. This text presents the latest applications of polyoxyalkylene block copolymers in areas such as medicine, coal and petroleum, plastics, emulsion polymerization, paper, photography, personal care and cleaner systems. It offers in-depth coverage of the subject from synthesis and analysis to toxicology and environmental impact.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Nonionic Surfactants est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Nonionic Surfactants par Vaughn Nace en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Sciences physiques et Chimie. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
1
Synthesis and Chemical Modification of Polyoxyalkylene Block Copolymers
I. | Polyether Overview |
A. General chemistry | |
B. Molecular weight (block size) considerations | |
C. Safety considerations | |
II. | Preparation and Characterization of Block Copolymers |
A. Polymer initiators | |
B. Base catalyst | |
C. Base neutralization | |
D. Polyether structural considerations | |
E. Planning the synthesis | |
F. Polymer characterization | |
III. | Synthetic Challenges |
A. Deviations from Flory’s first and second assumptions | |
B. Deviations from Flory’s third assumption | |
C. Other common synthetic problems | |
IV. | Modification of Polyoxyalkylene Block Copolymers |
A. Modification of the hydroxyl group | |
B. Modification of the polyether backbone | |
References |
I. POLYETHER OVERVIEW
A. General Chemistry
Polyethers are those polymers containing ether (C-O-C) linkages in the chain backbone. Such polymers are quite numerous. They are derived from a wide variety of monomers, by many different synthetic routes. Polyethers find utility in diverse applications either directly or as chemical intermediates [1]. of necessity this chapter deals with a limited subset of the total polyether realm. Only those polyethers derived from oxirane (also referred to as ethylene oxide and EO), methyloxirane (propylene oxide and PO), and ethyloxirane (1,2–butylene oxide and BO) will be discussed. Structures of these monomers are shown in Fig. 1. As a class these monomers are called oxiranes, alkylene oxides, AOs, or simply oxides.
While much of what will be said applies to all polyethers, the emphasis of this chapter follows the surfactant theme of the book and deals mainly with the synthesis and, to a lesser degree, the modification of block copolymers. Although many different catalyst systems are used for oxide polymerization [2], only the base-catalyzed mechanism is discussed here. The decision to focus only on preparations using base catalysis is appropriate, as strong base is the catalyst system used for most polyoxyalkylene block copolymers offered as surfactants by industry today [3,4]. Other catalysts used today cover a range of compositions including Lewis acids [5, 6, 7], metal coordination catalysts [8, 9, 10], and metal porphyrin [11, 12, 13]. Some of these catalyst systems readily provide stereoregular polyalkylene glycols of PO and BO [14,15]. Base catalyst is able to do so only when pure l or d oxide isomers are used [16]. While this is an interesting area, no more will be said about stereoregular polyalkylene glycols in this chapter.
Various drawbacks are found in every catalyst system used to date. The reader is encouraged to consider alternate synthetic methods which overcome the problems associated not only with base catalyst discussed below but also to become familiar with the other systems and the problems associated with each of them.
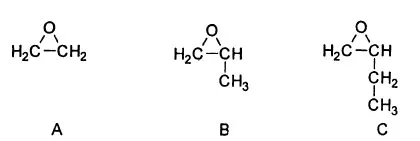
FIG. 1 Oxiranes of interest. A) Ethylene oxide. B) Propylene oxide. C) 1,2–Butylene oxide.
The base-catalyzed polymerization of alkylene oxides has been known for over a century [17,18]. Industrial applications emerged in the late 1940s [19,20]. Although this chapter is not intended to be all inclusive, it is the author’s wish that it be of benefit to surfactant scientists. For those workers who are now discovering the many uses of block copolymers as surfactants the chapter will serve as an introduction to the fundamental chemistry of alkylene oxide polymers. Appreciation of the many examples of how synthesis can go awry will help all workers. Remembering these anomalies will help to explain erratic results from the measurement of surfactant behavior.
Oxiranes are heterocyclic compounds consisting of two carbon and one oxygen atoms in the ring. All such rings are readily opened by a number of reagents. However, many highly substituted oxiranes do not undergo polymerization or do so only with difficulty. At the other extreme EO, PO, and BO polymerize readily with either acid or base catalysis. Commercial polyethers of low molecular weight (less than 20,000 daltons), narrow molecular weight dispersity, and based on these three oxides are prepared using strong base to facilitate the reaction. Base-catalyzed polymerization of alkylene oxides involves nucleophilic attack of an initiator molecule on one carbon of the ring resulting in hetero-bond cleavage. This bond cleavage generates an alkoxide anion. Itself an excellent nucleophile, the alkoxide anion reacts with another oxide molecule, thus propagating polyether chain growth. This is a second-order nucleophilic replacement (SN2) reaction [21]. The substitution reaction is useful for polymerization as the leaving group is not completely released from the molecule. In this way the oxiranes are bifunctional. Figure 2 depicts this propagation reaction; the formation of secondary hydroxyl groups (Reaction A) is greatly favored over primary hydroxyl groups (Reaction B) when propylene oxide and butylene oxide are polymerized under basic conditions [22]. This has been well known for many years. The implications of this selectivity will be discussed further below.
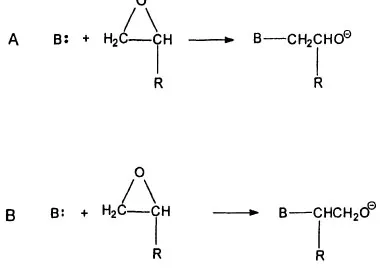
FIG. 2 Polymerization by SN2 propagation step. R = H, Me, or Et. Polymerizing ethylene oxide, R = H, reactions A and B are identical. With PO and BO, the reaction forming a secondary hydroxyl group (Reaction A) is greatly favored over formation of a primary hydroxy group (Reaction B). Secondary to primary ratio exceeds ten to one. Source: from [22].
When low molecular weights are involved, this system appears to be a living polymerization [23]. This pseudoliving process allows ready preparation of block copolymers. This is accomplished by reacting different monomers sequentially. Discrete blocks are formed when the concentration of the first oxide is reduced to essentially zero before the introduction of the second oxide. A transition zone containing both monomer units results when the second oxide is introduced before the first has completely reacted. Polyethers with random distributions of monomer units are made by adding different oxides together [24]. Deviation from a true living polymerization is discussed later in the chapter.
Polyoxyalkylene block copolymers are efficient surfactants. In these materials EO block(s) provide hydrophilicity and PO (or BO) block(s) the hydrophobicity necessary for surfactancy [25,26].
B. Molecular Weight (Block Size) Considerations
Polymerization of oxiranes under basic conditions does not proceed indefinitely. Molecular weights of polyethers prepared with a strong base catalyst might approach 20,000 daltons if a molecule with multiple nucleophilic sites is used as the initiating species. With a single site, however, the molecular weight will seldom exceed 5,000 daltons [27,28]. This molecular weight limit is imposed chemically by extraneous introduction of molecules which initiate growth of new polymer chains [29]. From a practical standpoint, molecular weights for industrial processes are limited by increasingly smaller polymerization rates due to a decrease in catalyst concentration as the polymerization proceeds.
Molecular weight distributions in polyethers are generally very narrow. Polydispersities of 1.05 to 1.15 are typical for polyethylene oxides [21] and slightly wider for polypropylene and polybutylene oxides ...