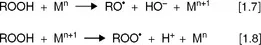Abstract:
Oxidative rancidity, one of the major causes of quality deterioration in foods, is caused by the oxidative deterioration of lipids by atmospheric oxygen. Lipids oxidize through a complex series of reactions giving rise to a myriad of non-volatile and volatile compounds that are responsible for off-flavours even at concentrations in the parts-per-billion range. This chapter provides a general overview of lipid oxidation in foods by presenting the main aspects of the oxidative development in general and, because oxidation of lipids is a complex process, for particular foods. The reaction mechanism and the principal factors affecting the rate of lipid oxidation are described. Then, the analytical methods normally applied to determine the extent of lipid oxidation, as well as accelerated methods to determine oxidative stability are presented. Finally, some recommendations are given on how to prevent or retard lipid oxidation.
1.1 Introduction: oxidative rancidity and food quality
Oxidative rancidity in foods refers to the perception of objectionable flavours and odours caused by oxidation of the unsaturated fatty acid chains of lipids by atmospheric oxygen. Because of the ‘spontaneous’ nature of the reaction the process is frequently referred to as autoxidation. Lipids oxidize through a complex series of reactions giving rise to a myriad of non-volatile and volatile compounds that are responsible for off-flavours even at concentrations in the parts-per-billion range. Lipid oxidation not only affects the quality of foods with impaired flavours and odours, but also with loss of essential nutrients such as fatty acids and vitamins, and with changes in texture and colour as a consequence of reactions of lipid oxidation products with other food components. It constitutes one of the major causes of quality deterioration in both natural and processed foods. Oxidation takes place at different stages of food preparation – in the raw materials, processing and packaging – and during storage. The principal consequence of the first events of lipid oxidation is a decrease in the shelf life of the food. Then, as oxidation progresses the development of flavours contributes significantly to impairing the sensorial quality until the food becomes unacceptable to the consumer.
In previous decades, enormous attention has been given to oxidized lipids because increased lipid peroxidation in vivo has been found in numerous degenerative and chronic diseases, mainly in cardiovascular diseases and cancer. In addition, a variety of lipid oxidation products present in foods has shown toxicity in both in vitro and in vivo studies (Esterbauer et al., 1990; Kubow, 1992; Kanazawa et al., 2002). Nevertheless, the extent to which oxidized lipids in foods contribute to the pathogenesis of diseases is at present unknown. One of the principal reasons is because the information available on the structures and contents of oxidation products in foods is rather scant. It is known that from a quantitative point of view fried foods constitute the main source of oxidized lipids in our diet. During the frying process the oil is subjected to high temperatures that accelerate the formation of oxidation products, which are then along with the frying oil incorporated to the food. For foods that have been subjected to ambient or moderate temperatures, oxidation products do not normally reach more than 5 wt % of the total fat content, since at this level rancidity can be readily detected. On the contrary, oxidized lipids are in part responsible for the appreciated flavour in fried foods and for this reason their level can be rather high (Dobarganes and Márquez-Ruiz, 2003). In most countries having regulation on used frying oils, the degradation limit established for human consumption is 25 wt % (Firestone, 1996).
Lipids are present in practically all foodstuffs with the major classes being triacylglycerols (also named triglycerides), which occur in fat storage cells of plants and animals, and, to a lower extent, phospholipids, which occur in biological membranes. Oxidation mainly takes place in the chains of unsaturated fatty acids of triglycerides and phospholipids, oleate, linoleate and linolenate being the most abundant unsaturated fatty acids in the diet.
Oxidative deterioration is a classical problem of great economic concern in the food industry as it affects many foods irrespective of the fat content. Thus, organoleptically detectable lipid oxidation can occur in foods having 0.5% fat content or even lower (Fritsch, 1994).
There is a trend towards incorporating into foods nutritionally functional lipids containing fatty acids with two or more double bonds, such as conjugated linoleic acid, α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These lipids are readily oxidisable substrates and their utilization thus shortens significantly the shelf life of the supplemented food and can even give rise to uncontrolled oxidation problems (Kolanowsky et al., 2007).
This chapter presents a general overview of lipid oxidation in foods by presenting the main aspects of the oxidative development in general and, because oxidation of lipids is a complex process, for particular foods. The reaction mechanism and the principal factors affecting the rate of lipid oxidation are described. Then, the analytical methods normally applied to determine the extent of lipid oxidation, as well as accelerated methods to determine oxidative stability are presented. Finally, some recommendations on how to prevent or retard lipid oxidation are given.
1.2 Mechanisms of lipid oxidation
1.2.1 Autoxidation
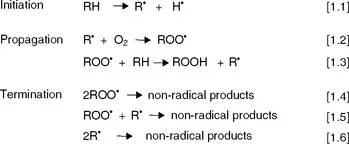
Autoxidation of lipids takes place through chain reactions of free radicals following an overall mechanism that consists of three stages: initiation, propagation and termination (Fig. 1.1). In the initiation stage, an alkyl radical is formed by abstraction of a hydrogen radical from an allylic position, equation [1.1]. In the propagation step, the alkyl radical reacts with oxygen at rates controlled by diffusion to form peroxyl radicals, equation [1.2] that, in turn, react with new lipid molecules giving rise to hydroperoxides as the primary oxidation products and new alkyl radicals that propagate the reaction chain, equation [1.3]. Finally, in the termination stage, radicals react between each other to yield relatively stable non-radical species, equations [1.4]–[1.6].
Fig. 1.1 Mechanism of lipid autoxidation.
The mechanism of initiation has been a subject of debate for many years. Equation [1.1] is the most accepted initiation reaction. However, the hydrogen radical is not released spontaneously from the lipid molecule, but it is abstracted by initiators. Ubiquitous hydroperoxides and trace heavy metals, both always present as impurities in lipids, seem to have an important role in the generation of radicals that act as initiators. Minor contents of hydroperoxides, mainly produced by enzymatic oxidation or by photoxidation, decompose into radicals through metal catalysis according to equations [1.7] and [1.8] (Fig. 1.2), and these are thought to be involved in the first reactions of hydrogen abstraction (Frankel, 2005).
Fig. 1.2 Metal-catalyzed decomposition of hydroperoxides.
After the formation of the first radicals, the three stages of oxidation occur simultaneously, but at varying rates that change during the process. It is known that the step determining the rate is the propagation reaction, equation [1.3], which leads to accumulation of hydroperoxides by the reaction of peroxyl radicals with lipid substrate molecules. As a result, hydrogen abstraction from unsaturated lipids is selective for the most weakly bound hydrogens. The susceptibility of lipids to oxidation thus depends on the availability of allylic hydrogens becau...