
eBook - ePub
Chemical Deterioration and Physical Instability of Food and Beverages
- 824 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemical Deterioration and Physical Instability of Food and Beverages
About this book
For a food product to be a success in the marketplace it must be stable throughout its shelf-life. Quality deterioration due to chemical changes and alterations in condition due to physical instability are not always recognised, yet can be just as problematic as microbial spoilage. This book provides an authoritative review of key topics in this area.
Chapters in part one focus on the chemical reactions which can negatively affect food quality, such as oxidative rancidity, and their measurement. Part two reviews quality deterioration associated with physical changes, such as moisture loss, gain and migration, crystallization and emulsion breakdown. Contributions in the following section outline the likely effects on different foods and beverages, including bakery products, fruit and vegetables, ready-to-eat meals and wine.
With contributions from leaders in their fields, Chemical deterioration and physical instability of food and beverages is an essential reference for R&D and QA staff in the food industry and researchers with an interested in this subject.
- Examines chemical reactions which can negatively affect food quality and measurement
- Reviews quality deterioration associated with physical changes such as moisture loss, gain and migration, and crystallization
- Documents deterioration in specific food and beverage products including bakery products, frozen foods and wine
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Chemical Deterioration and Physical Instability of Food and Beverages by Leif H Skibsted,Jens Risbo,Mogens L Andersen in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Food Industry. We have over one million books available in our catalogue for you to explore.
Information
Part I
Understanding and measuring chemical deterioration of food and beverages
1
Oxidative rancidity in foods and food quality
J. Velasco, C. Dobarganes and G. Márquez-Ruiz, Consejo Superior de Investigaciones Científicas (CSIC), Spain
Abstract:
Oxidative rancidity, one of the major causes of quality deterioration in foods, is caused by the oxidative deterioration of lipids by atmospheric oxygen. Lipids oxidize through a complex series of reactions giving rise to a myriad of non-volatile and volatile compounds that are responsible for off-flavours even at concentrations in the parts-per-billion range. This chapter provides a general overview of lipid oxidation in foods by presenting the main aspects of the oxidative development in general and, because oxidation of lipids is a complex process, for particular foods. The reaction mechanism and the principal factors affecting the rate of lipid oxidation are described. Then, the analytical methods normally applied to determine the extent of lipid oxidation, as well as accelerated methods to determine oxidative stability are presented. Finally, some recommendations are given on how to prevent or retard lipid oxidation.
Key words
lipid oxidation
oxidative stability
quality deterioration
1.1 Introduction: oxidative rancidity and food quality
Oxidative rancidity in foods refers to the perception of objectionable flavours and odours caused by oxidation of the unsaturated fatty acid chains of lipids by atmospheric oxygen. Because of the ‘spontaneous’ nature of the reaction the process is frequently referred to as autoxidation. Lipids oxidize through a complex series of reactions giving rise to a myriad of non-volatile and volatile compounds that are responsible for off-flavours even at concentrations in the parts-per-billion range. Lipid oxidation not only affects the quality of foods with impaired flavours and odours, but also with loss of essential nutrients such as fatty acids and vitamins, and with changes in texture and colour as a consequence of reactions of lipid oxidation products with other food components. It constitutes one of the major causes of quality deterioration in both natural and processed foods. Oxidation takes place at different stages of food preparation – in the raw materials, processing and packaging – and during storage. The principal consequence of the first events of lipid oxidation is a decrease in the shelf life of the food. Then, as oxidation progresses the development of flavours contributes significantly to impairing the sensorial quality until the food becomes unacceptable to the consumer.
In previous decades, enormous attention has been given to oxidized lipids because increased lipid peroxidation in vivo has been found in numerous degenerative and chronic diseases, mainly in cardiovascular diseases and cancer. In addition, a variety of lipid oxidation products present in foods has shown toxicity in both in vitro and in vivo studies (Esterbauer et al., 1990; Kubow, 1992; Kanazawa et al., 2002). Nevertheless, the extent to which oxidized lipids in foods contribute to the pathogenesis of diseases is at present unknown. One of the principal reasons is because the information available on the structures and contents of oxidation products in foods is rather scant. It is known that from a quantitative point of view fried foods constitute the main source of oxidized lipids in our diet. During the frying process the oil is subjected to high temperatures that accelerate the formation of oxidation products, which are then along with the frying oil incorporated to the food. For foods that have been subjected to ambient or moderate temperatures, oxidation products do not normally reach more than 5 wt % of the total fat content, since at this level rancidity can be readily detected. On the contrary, oxidized lipids are in part responsible for the appreciated flavour in fried foods and for this reason their level can be rather high (Dobarganes and Márquez-Ruiz, 2003). In most countries having regulation on used frying oils, the degradation limit established for human consumption is 25 wt % (Firestone, 1996).
Lipids are present in practically all foodstuffs with the major classes being triacylglycerols (also named triglycerides), which occur in fat storage cells of plants and animals, and, to a lower extent, phospholipids, which occur in biological membranes. Oxidation mainly takes place in the chains of unsaturated fatty acids of triglycerides and phospholipids, oleate, linoleate and linolenate being the most abundant unsaturated fatty acids in the diet.
Oxidative deterioration is a classical problem of great economic concern in the food industry as it affects many foods irrespective of the fat content. Thus, organoleptically detectable lipid oxidation can occur in foods having 0.5% fat content or even lower (Fritsch, 1994).
There is a trend towards incorporating into foods nutritionally functional lipids containing fatty acids with two or more double bonds, such as conjugated linoleic acid, α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These lipids are readily oxidisable substrates and their utilization thus shortens significantly the shelf life of the supplemented food and can even give rise to uncontrolled oxidation problems (Kolanowsky et al., 2007).
This chapter presents a general overview of lipid oxidation in foods by presenting the main aspects of the oxidative development in general and, because oxidation of lipids is a complex process, for particular foods. The reaction mechanism and the principal factors affecting the rate of lipid oxidation are described. Then, the analytical methods normally applied to determine the extent of lipid oxidation, as well as accelerated methods to determine oxidative stability are presented. Finally, some recommendations on how to prevent or retard lipid oxidation are given.
1.2 Mechanisms of lipid oxidation
1.2.1 Autoxidation
Autoxidation of lipids takes place through chain reactions of free radicals following an overall mechanism that consists of three stages: initiation, propagation and termination (Fig. 1.1). In the initiation stage, an alkyl radical is formed by abstraction of a hydrogen radical from an allylic position, equation [1.1]. In the propagation step, the alkyl radical reacts with oxygen at rates controlled by diffusion to form peroxyl radicals, equation [1.2] that, in turn, react with new lipid molecules giving rise to hydroperoxides as the primary oxidation products and new alkyl radicals that propagate the reaction chain, equation [1.3]. Finally, in the termination stage, radicals react between each other to yield relatively stable non-radical species, equations [1.4]–[1.6].
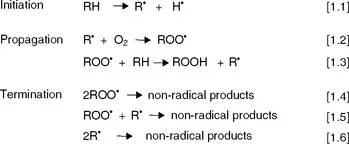
Fig. 1.1 Mechanism of lipid autoxidation.
The mechanism of initiation has been a subject of debate for many years. Equation [1.1] is the most accepted initiation reaction. However, the hydrogen radical is not released spontaneously from the lipid molecule, but it is abstracted by initiators. Ubiquitous hydroperoxides and trace heavy metals, both always present as impurities in lipids, seem to have an important role in the generation of radicals that act as initiators. Minor contents of hydroperoxides, mainly produced by enzymatic oxidation or by photoxidation, decompose into radicals through metal catalysis according to equations [1.7] and [1.8] (Fig. 1.2), and these are thought to be involved in the first reactions of hydrogen abstraction (Frankel, 2005).
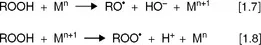
Fig. 1.2 Metal-catalyzed decomposition of hydroperoxides.
After the formation of the first radicals, the three stages of oxidation occur simultaneously, but at varying rates that change during the process. It is known that the step determining the rate is the propagation reaction, equation [1.3], which leads to accumulation of hydroperoxides by the reaction of peroxyl radicals with lipid substrate molecules. As a result, hydrogen abstraction from unsaturated lipids is selective for the most weakly bound hydrogens. The susceptibility of lipids to oxidation thus depends on the availability of allylic hydrogens becau...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Woodhead Publishing Series in Food Science, Technology and Nutrition
- Introduction
- Part I: Understanding and measuring chemical deterioration of food and beverages
- Part II: Understanding and measuring physical deterioration of foods and beverages
- Part III: Deterioration in specific food and beverage products
- Index