
Significant Pharmaceuticals Reported in US Patents
Thomas F. DeRosa
- 700 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
Significant Pharmaceuticals Reported in US Patents
Thomas F. DeRosa
À propos de ce livre
Significant Pharmaceuticals Reported in US Patents identifies the next generation of pharmaceuticals reported in US Patents. This "hands-on" title provides explicit laboratory methods for preparing the most recent and effective medications. Each entry documents the biological testing protocols used to evaluate a drug and the significance of the current treatment agent over previous methods. Pharmaceuticals are included in this review only if at least two of the following criteria were met: Effectiveness in treating an illness, Innovative, ease of preparation, synergy with existing Medications.
Pharmaceuticals are reported for 27 separate classes of illness, including: AIDS, Alzheimer's Disease, Cardiovascular Disorders, Diabetes, Epilepsy, Hepatitis C, Osteoporosis, Obesity and Sleep Disorders.
Significant Pharmaceuticals Reported in US Patents has been designed to be used as both a reference and synthetic guide for pharmaceutical, medicinal and organic chemists and graduate students.
Researchers working in other areas will also find the information valuable as in many instances intermediates or the next generation pharmaceutical are readily convertible into other industrial products including: anti-oxidants, chemical additives, herbicides, polymer precursors, water purification agents. Clear structural depictions of reagents and chemical transformations have been supplied to permit the identification of other future applications.
- Identifies next generation pharmaceuticals
- Provides practical preparation methods for each active agent and derivatives
- Documents the analytical characterization and biological testing results of active agents
Foire aux questions
Informations
Acquired Immune Deficiency Syndrome
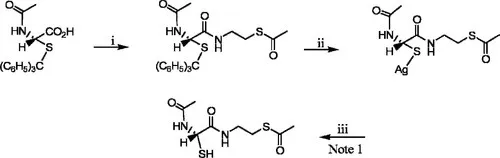
J. Oiry et al., US Patent 6,989,372 (January 24, 2006)
Reaction

Experimental
A solution of N-acetyl-S-trityl-l-cysteine (0.71 mmol) and 80μ1 N-methylmorpholine dissolved in 5 ml of EtOAc was stirred at–15°C, then treated with 93μl isobutyl chloroformate. After 15 minutes, S-acetylcysteamine hydrochloride (0.71 mmol) and an additional 80μl N-methyl-morpholine were added and the mixture stirred for 15 minutes at – 15°C and then 3 hours at ambient temperature. N-Methylmorpholine hydrochloride was then filtered off and the mixture was washed twice with 2.5 ml of EtOAc and concentrated. The gummy residue was purified by flash chromatography with silica gel using EtOAc/30% petroleum ether and the product isolated in 55% yield as a colorless powder, mp=111−113°C.
Rf = 0.41, EtOAc/petroleum ether, 9:1
[α]D20=+10.5° (c. 0.8, CHCl3
1H NMR (CDCl3) δ (ppm) 1.90 (s, 3H, NCOCH3), 2.29 (s, 3H, SCOCH3), 2.48 (dd, J = 5.7, 12.9Hz, 1H, β Ha cys), 2.82 (dd, J = 6.4, 12.9Hz, 1H, β Hb cys), 2.92–3.01 (m, 2H, NCH2CH2S), 3.32–3.42 (m, 2H, NCH2CH2S), 4.07–4.20 (m, 1H, α H cys), 5.70 (d, J = 7.6Hz, 1H, NH cys), 6.34 (t, J = 5.5Hz, 1H, NHCH2), 7.19–7.35 and 7.40–7.47 (2m, 15H, aromatic H)
MS (FAB +/NBA + K +) m/z 545 (M + K)+, 507 (M + H)+; (FAB-/NRA) m/z 505 (M-H)−
Analysis Calc. for C28H30N2O3S2 (506): C, 66.40; H, 5.93; N, 5.53. Found: C, 66.17; H, 6.00; N, 5.81