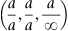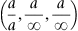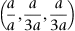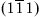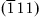Introduction to Dislocations
Derek Hull, D. J. Bacon
- 272 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
Introduction to Dislocations
Derek Hull, D. J. Bacon
À propos de ce livre
In materials science, dislocations are irregularities within the crystal structure or atomic scale of engineering materials, such as metals, semi-conductors, polymers, and composites. Discussing this specific aspect of materials science and engineering, Introduction to Dislocations is a key resource for students. The book provides students and practitioners with the fundamental principles required to understand dislocations. Comprised of 10 chapters, the text includes advanced computer modeling and very high-resolution electron microscopy to help readers better understand the structure of atoms close to the core of dislocations. It shows that atomic arrangement has a significant effect on the formation of dislocations and thereby on the properties of solids. The first two chapters of the book present an overview of dislocations. The crystal structures and the various defects and dislocations are discussed, and methods of observation and diagnosis of dislocations are covered. Chapters 3 to 5 discuss the behavior of dislocations and explain how changes in the structure and arrangement of atoms can affect the behavior of dislocations. The three chapters also discuss the mechanical properties of dislocations. The remaining chapters offer a detailed discussion of the mechanisms of dislocations and the mechanical strength of crystalline solids. The book is written for undergraduate- and graduate-level students in both materials science and mechanical engineering. Non-experts and novices working on mechanical properties, mechanisms of deformation and fracture, and properties of materials, as well as industrial and academic researchers, will find this book invaluable.
- Long-established academic reference by an expert author team, highly regarded for their contributions to the field.
- Uses minimal mathematics to present theory and applications in a detailed yet easy-to-read manner, making this an understandable introduction to a complex topic.
- Unlike the main competition, this new edition includes recent developments in the subject and up-to-date references to further reading and research sources.
Foire aux questions
Informations
1.1. Crystalline Materials
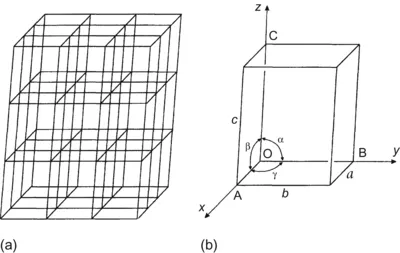 |
| Figure 1.1 (a) A space lattice, (b) unit cell showing positions of principal axes. |
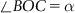
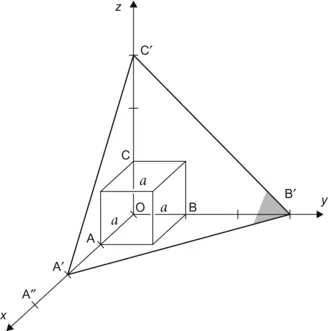 |
| Figure 1.2 Cubic cell illustrating method of describing the orientation of planes. |
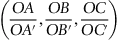


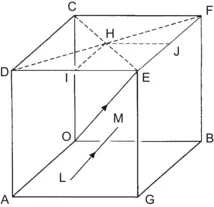 |
| Figure 1.3 Cubic cell illustrating the method of describing directions and point sites. LM is parallel to OE. |