1.1 Polymerization reaction
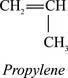
Polypropylene is prepared by polymerizing propylene, a gaseous byproduct of petroleum refining, in the presence of a catalyst under carefully controlled heat and pressure. [773] Propylene is an unsaturated hydrocarbon, containing only carbon and hydrogen atoms:
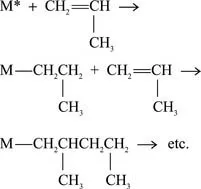
In the polymerization reaction, many propylene molecules (monomers) are joined together to form one large molecule of polypropylene. Propylene is reacted with an organometallic, transition metal catalyst (see 1.4 Catalysts for a description of catalysts used in the reaction) to provide a site for the reaction to occur, and propylene molecules are added sequentially through a reaction between the metallic functional group on the growing polymer chain and the unsaturated bond of the propylene monomer:
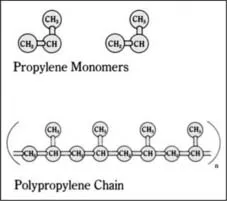
One of the double-bonded carbon atoms of the incoming propylene molecule inserts itself into the bond between the metal catalyst (M in the above reaction) and the last carbon atom of the polypropylene chain. A long, linear polymer chain of carbon atoms is formed, with methyl (CH3) groups attached to every other carbon atom of the chain (Figure 1.1). Thousands of propylene molecules can be added sequentially until the chain reaction is terminated. [764, 768]
1.2 Stereospecificity
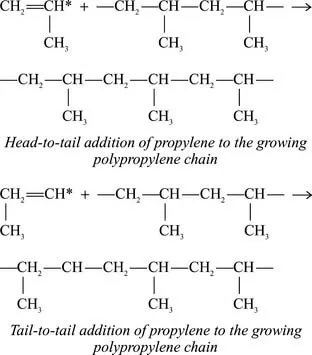
With Ziegler-Natta or metallocene catalysts, the polymerization reaction is highly stereospecific. Propylene molecules add to the polymer chain only in a particular orientation, depending on the chemical and crystal structure of the catalyst, and a regular, repeating three-dimensional structure is produced in the polymer chain [763]. Propylene molecules are added to the main polymer chain, increasing the chain length, and not to one of the methyl groups attached to alternating carbon atoms (the pendant methyl groups), which would result in branching. Propylene molecules are usually added head-to-tail and not tail-to-tail or head-to-head. Head-to-tail addition results in a polypropylene chain with pendant methyl groups attached to alternating carbons; in tail-to-tail or head-to head addition, this alternating arrangement is disrupted. [771]
Occasional tail-to-tail or head-to-tail additions of polypropylene to the growing polymer chain disrupt the crystalline structure and lower the melting point of the polymer; formulations in which this occurs are used in thermoforming or blow molding. [694]
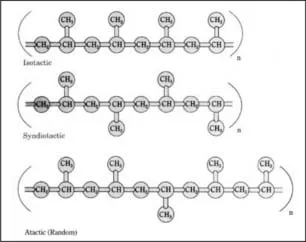
Polypropylene can be isotactic, syndiotactic, or atactic, depending on the orientation of the pendant methyl groups attached to alternate carbon atoms. In isotactic polypropylene (Figure 1.2), the most common commercial form, pendant methyl groups are all in the same configuration and are on the same side of the polymer chain. Due to this regular, repeating arrangement, isotactic polypropylene has a high degree of crystallinity. In syndiotactic polypropylene, alternate pendant methyl groups are on opposite sides of the polymer backbone, with exactly opposite configurations relative to the polymer chain. Syndiotactic polypropylene is now being produced commercially using metallocene catalysts. In atactic polypropylene, pendant methyl groups have a random orientation with respect to the polymer backbone. Amounts of isotactic, atactic, and syndiotactic segments in a formulation are determined by the catalyst used and the polymerization conditions. Most polymers are predominantly isotactic, with small amounts of atactic polymer. New metallocene catalysts make possible other stereochemical configurations, such as hemi-isotactic polypropylene. In this configuration, most pendant methyl groups are on the same side of the polypropylene chain, as in isotactic polypropylene; however, other methyl groups are inserted at regular intervals on the opposite side of the chain. [794, 695, 810]
1.3 Effect on characteristics of polypropylene
The structure and stereochemistry of polypropylene affect its properties.
1.3.1 Stereochemistry
Because of its structure, isotactic polypropylene has the highest crystallinity, resulting in good mechanical properties such as stiffness and tensile strength. Syndiotactic polypropylene is less stiff than isotactic but has better impact strength and clarity. Due to its irregular structure, the atactic form has low crystallinity, resulting in a sticky, amorphous material used mainly for adhesives and roofing tars. [794, 691] Increasing the amount of atactic polypropylene in a predominantly isotactic formulation increases the room temperature impact resistance and stretchability but decreases the stiffness, haze, and color quality. [695] The amount of atactic polypropylene in a polypropylene formulation is indicated by the level of room temperature xylene solubles; levels range from about 1–20%. [771] Polypropylenes generally have...