1.1 INHALATION ANESTHETICS
The object of inhalation anesthetics is to obtain a concentration (partial pressure) of the drug in the brain sufficient to reach the desired level of anesthesia. In order to do this, anesthetic molecules must pass through the lungs into the brain through various biological phases. Therefore, inhalation anesthetics must be soluble in blood and interstitial tissue.
The wide variation in structure, ranging from complex steroids to the inert monatomic gas xenon, led to several theories of anesthetic action. The mechanism by which inhalation anesthetics manifest their effect is not exactly known. Since they do not belong to one chemical class of compounds, the correlations between structure and activity are also not known. Inhalation anesthetics are nonspecific and therefore there are not specific antagonists. Interaction of inhalation anesthetics with cellular structures can only be described as van der Waals interactions. There are a number of hypotheses that have been advanced to explain the action of inhalation anesthetics; however, none of them can adequately describe the entire spectrum of effects caused by inhalation anesthetics.
The action of general anesthetics can be explained as a blockage of ion channels, or as specific changes in mechanisms of the release of neurotransmitters. Three of the proposed mechanisms are mentioned below.
1. Hydrate hypothesis: Anesthetic molecules can form hydrates with structured water, which can stop brain function in corresponding areas. However, the correlation between the ability to form hydrates and the activity of inhalation anesthetics is not known.
2. Ion channel hypothesis: Anesthetics block ion channels by interacting with cellular membranes and reducing the flow of Na+ ions and increasing the flow of K+ ions into the cell, which leads to the development of anesthesia.
3. Fluid membrane hypothesis: Anesthetics stabilize, or rather immobilize the cell membrane, hampering membrane fluidity, which produces changes in the ion channel action.
Selection of a specific anesthetic or combination of anesthetics is made depending on the type of medical intervention. For a long time, ether, chloroform, tricholoroethylene, ethyl chloride or chloretane, and also cyclopropane were widely used as inhalation anesthetics. Today, the following anesthetics are used most regularly in medicine: halothane, enflurane, isoflurane, metoxyflurane, and nitrous oxide. Researchers are also actively exploring the use of xenon as an anesthetic.
Halothane
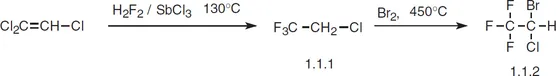
Halothane, 2-bromo-2-chloro-1,1,1-trifluorethane (1.1.2), is made by the addition of hydrogen fluoride to tricholoroethylene and simultaneous substitution of chlorine atoms in the presence of antimony(III) chloride at 130 °C. The resulting 2-chloro-1,1,1-trifluorethane (1.1.1) undergoes further bromination at 450 °C to form halothane [1–3].
Halothane is a modern and widely used inhalation anesthetic. It begins to act very quickly, which is pleasing to patients, and it is very safe. The only drawback to using it is its hepatotoxicity. It is used in both short and long-lasting surgical operations. The most common synonym of halothane is fluothane.
Enflurane
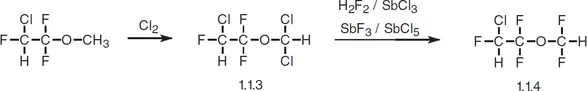
Enflurane, 2-chloro-1,1,2-trifluoroethyldifluoromethyl ether (1.1.4), is synthesized by chlorinating in light 2-chloro-1,1,2-trifluoroethylmethyl ether to give 2-chloro-1,1,2-trifluoroethyldichloromethyl ether (1.1.3), followed by substitution of chlorine atoms by fluorine on the dichloromethyl group using hydrogen fluoride in the presence of antimony(III) chloride, or by using antimony(III) fluoride with antimony(V) chloride [4,5].
Enflurane has practically all the same characteristics as halothane and is used in the same situations. It is poorly absorbed. It is also prescribed under the name ethrane.
Isoflurane
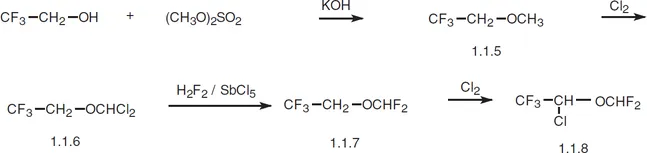
Isoflurane, 2-chloro-2-(difluoromethoxy)-1,1,1-trifluorethane (1.1.8), is synthesized from 2,2,2-trifluoroethanol. 2,2,2-Trifluoroethanol is first methylated by dimethylsulfate. The resulting methyl ether (1.1.5) undergoes chlorination by molecular chlorine to give 2-(dichloromethoxy)-1,1,1-trifluoroethane (1.1.6). In the subsequent interaction (1.1.6) with hydrogen fluoride in the presence of antimony(V) chloride, chlorine atoms are ultimately replaced by fluorine atoms. The resulting ether (1.1.7) again undergoes chlorination by molecular chlorine to give isoflurane [6,7].
In terms of action, isoflurane is analogous to enflurane; howe...