
eBook - ePub
Enzyme Kinetics and Mechanism
Paul F. Cook, W. W. Cleland
This is a test
Partager le livre
- 416 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Enzyme Kinetics and Mechanism
Paul F. Cook, W. W. Cleland
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Enzyme Kinetics and Mechanism is a comprehensive textbook on steady-state enzyme kinetics. Organized according to the experimental process, the text covers kinetic mechanism, relative rates of steps along the reaction pathway, and chemical mechanism—including acid-base chemistry and transition state structure.
Practical examples taken from the literature demonstrate theory throughout. The book also features numerous general experimental protocols and how-to explanations for interpreting kinetic data.
Written in clear, accessible language, the book will enable graduate students well-versed in biochemistry to understand and describe data at the fundamental level. Enzymologists and molecular biologists will find the text a useful reference.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Enzyme Kinetics and Mechanism est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Enzyme Kinetics and Mechanism par Paul F. Cook, W. W. Cleland en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Medicine et Biochemistry in Medicine. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
1
NOMENCLATURE
An understanding of the language of steady-state kinetics is an important prerequisite to an understanding of the theory and experimental approaches employed by the kineticist. This chapter is devoted to a discussion of the nomenclature developed by Cleland (BBA 67, 104).
Reaction Components
Enzymes catalyze chemical reactions with specific reactants that are called substrates and products. Rather than referring to the actual name of the reactants, a convenient shorthand notation has been developed in which reactants, inhibitors, activators, and enzyme are replaced by letters. Substrates are designated by the letters A, B, C, and D in the order to which they add to enzyme, and products are designated P, Q, R, and S in the order that they are released from the enzyme surface. To illustrate, the alcohol dehydrogenase reaction is shown in equation 1-1, where A and B represent NAD+ and ethanol, and P and Q represent acetaldehyde and NADH:
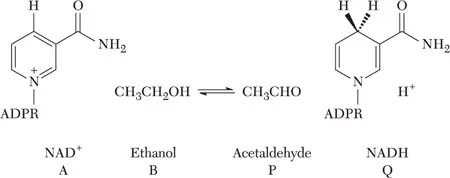 | (1-1) |
A metal ion activator can be designated by M and the ionic charge or by the actual chemical symbol for the metal ion, for exampe, M2+ or Mg2+, while other activators are designated by X, Y, and Z. The letters E, F, and G are reserved for enzyme forms as will be discussed further. The product of an enzyme-catalyzed reaction is a substrate in the opposite direction for a reversible reaction. Thus, the physiologic reaction direction (if known) is chosen for substrates, but it is always best to specify the reaction direction. Inhibitors are designated I and J. The following two reactions schematically depict enzyme combining with substrate to generate product and the combination of enzyme and inhibitor, respectively:
(1-2) |
(1-3) |
There are two kinds of enzyme forms called stable and transitory. An enzyme form is termed stable if, when isolated from the rest of the reaction components, it has a long half-life with respect to the assay time scale, and reactants add to it in a bimolecular step. Stable enzyme forms that occur in a single reaction cycle, that is, conversion of one molecule of reactant to product, are given the abbreviation E, F, or G. An enzyme form is termed transitory if, when isolated from the rest of the reaction components, its half-life is short relative to the assay time scale. The latter are usually enzyme–reactant complexes. In equations 1-2 and 1-3, E is a stable enzyme form while EA, EP, and EI are transitory enzyme forms, since they form and decompose rapidly. Consider the examples given in equations 1-4 and 1-5, where E and F are stable enzyme forms and EA, FP, FB, EQ, EAB, and EPQ are transitory enzyme forms. Differences between E and F will be discussed in detail later, but they are chemically and/or structurally different forms of the enzyme.
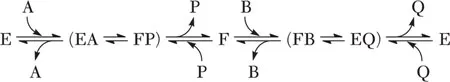 | (1-4) |
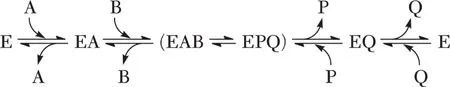 | (1-5) |
There are two types of transitory enzyme complexes, those in which the active site is fully occupied by substrates and products and those in which it is partially filled. Transitory complexes in which the active site is completely filled and kinetically competent are called central complexes, while the others are termed noncentral. The transitory enzyme complexes are denoted by E and the bound reactant, for example, EA and EQ. Central complexes are sometimes additionally enclosed in parentheses, for example, (EAB) and (EPQ), or with both reactants in parentheses along with arrows to show the interconversion of EAB and EPQ. Complexes with one, two, or three reactants and/or products bound are called binary, ternary, and quaternary. In equation 1-4, EA, FP, FB, and EQ are binary central complexes, while in equation 1-5, EAB and EPQ are ternary central complexes. Noncentral complexes are not present in equation 1-4, while EA and EQ are noncentral transitory complexes in equation 1-5.
Kinetic Mechanism
Kinetic mechanisms, that is, the order of addition of reactants to and release of products from the enzyme active site, fall into two classes. Reactions in which all reactants must be bound to an enzyme before any reaction occurs are called sequential, while those in which a product is released between the addition of two substrates are called ping-pong.
A sequential kinetic mechanism is depicted in equation 1-5; both A and B are bound to E before conversion to P and Q takes place. For this type of reaction there is usually only a single stable enzyme form present, E. Sequential mechanisms can be termed either ordered, when there is an obligatory order of addition of reactants to an enzyme and/or release of products from enzyme, or random, when there is not. The term rapid equilibrium is used to indicate a special case in which the equilibrium between an enzyme–reactant complex and free reactant is established in a reaction cycle. The latter will occur when subsequent steps are slow compared to the rate of dissociation of reactant.
The example shown in equation 1-4 depicts a ping-pong kinetic mechanism in which P is released prior to the addition of B. Two stable enzyme forms are present in this type of reaction, and these enzyme forms can usually be isolated. In the ping-pong mechanism a reactant binds to the enzyme active site, undergoes a chemical transformation, and leaves a fragment (or part of the substrate) on the enzyme prior to dissociating as a product. The second reactant then binds, undergoes a second chemical transformation...