
Frontiers in Clinical Drug Research - Dementia
José Juan Antonio Ibarra Arias
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
Frontiers in Clinical Drug Research - Dementia
José Juan Antonio Ibarra Arias
À propos de ce livre
Among neurodegenerative diseases, those that lead to a state ofdementia are the aim of severalinvestigations. Dementia is a chronic disease the prevalence of whichis increasing worldwide. Thenumber of dementia patients in the world is approximately 50 million, and it is estimated that thenumber of patients will reach 131.5 million by 2050. This increase willbe accompanied by asignificant increase in medical expenditures and other expenses, especially for elderly patients.Therefore, the maintenance cost of dementia in the future is expectedto be quite high. For thisreason, several investigations aim, firstly, to describe the keymechanisms involved in the originof dementia and, secondly, to establish preventive and therapeuticstrategies in order tounderstand and mitigate this debilitating pathology. This volume of Frontiers in Clinical Drug Research -Dementia explores the current comorbidities that cause cognitiveimpairment and the current management alternatives for clinical cases ofdementia. The reviews contributed in these volume will provide readers with acurrent perspective on the subject. The topics covered in this volume include: - Comorbidities inducing mild cognitive impairment - an evaluation ofthe risk caused by some pathological conditions- Tau-targeted therapy in Alzheimer's disease - history and currentstate- Emerging nanotherapeutic strategies in Alzheimer's disease- Implication of dehydroepiandrosterone on dementia related tooxidative stress- Polyphenol compounds as potential therapeutic agents in Alzheimer'sdisease The volume is a timely update on dementia treatment for clinicalphysicians, neurologists, gerontologists, pharmaceutical and medicinal chemistryresearchers, and physiologists.
Foire aux questions
Informations
Tau-Targeted Therapy in Alzheimer's Disease - History and Current State
Anamaria Jurcau1, *, Vharoon Sharma Nunkoo1
Abstract
* Corresponding author Anamaria Jurcau: The University of Oradea, Faculty of Medicine and Pharmacy, Oradea, Romania; E-mail:[email protected]
HISTORICAL BACKGROUND
NORMAL TAU PROTEIN STRUCTURE AND FUNCTION
The Tau Gene and Tau Isoforms
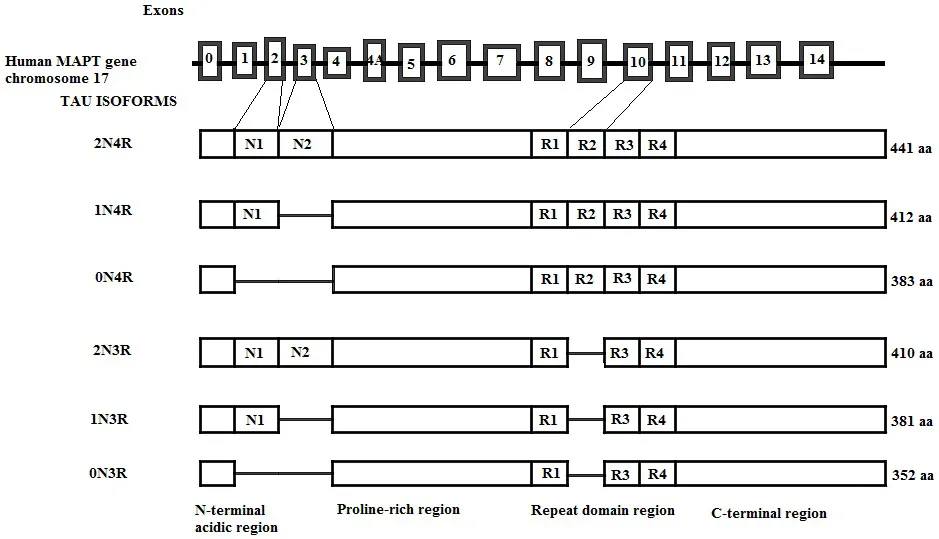
Genomic structure of the human tau gene; of the 14 exons, exons 2, 3, and 10 are alternatively spliced, generating six tau isoforms in the adult brain. Exons 9, 11, and 12 each encode for a microtubule-binding repeat generating 3R tau isoforms. The presence of E10 adds an extra MT-binding repeat generating 4R tau isoforms. 3R and 4R tau isoforms further differ depending on the presence of exon 2 (1N) or exons 2 and 3 together (2N), while the absence of both exons generates 0N3R and 0N4R isoforms of tau. The number of aminoacids in each isoform is shown on the right.
Tau Protein Structure

In the free tau folded into the paperclip conformation, the C-terminal domain lies close to the N-terminus and protects the domain comprising aminoacids 2-18, known as the phosphatase activating domain to interact with protein phosphatase 1 (PP1). When the C-terminal domain moves away from the N-terminal domain, the phosphatase activating domain activates PP1, which activates further glycogen synthase kinase 3β (GSK3β) and initiates the phosphorylation cascade.
Tau Localization
Tau Localization in Neurons
- cytosolic tau diffuses freely between the different compartments but is retained in the axon through its binding to microtubules favored by the low phosphorylation level and the retrograde barrier formed by the initial segment of the axon, which “traps” tau in the axon [25].
- tau is transported actively by motor pr...