![]()
CHAPTER 1
Polymerization in Ionic Liquids
NIKHIL K. SINGHAa, KUNLUN HONGb AND JIMMY W. MAYS*c
a Rubber Technology Centre, Indian Institute of Technology, Kharagpur 721302, India
b Center for Nanophase Materials Sciences, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
c Department of Chemistry, University of Tennessee, Knoxville, TN 37996, USA
1.1 Introduction
Most liquids used as solvents are composed of neutral molecules. In contrast, ionic liquids (ILs) are salts in the liquid state at ambient or near ambient temperatures. This room temperature ionic liquid state is often achieved by choosing ion pairs where one is organic and has a delocalized charge, or by choosing bulky asymmetric substituents. This causes the ions to be poorly coordinated, resulting in low melting temperatures. In principle, literally millions of ionic liquids with an exceptionally wide range of properties can be produced. This has led to ILs being considered as designer solvents or task specific solvents, where their extremely low vapor pressures offer potential to minimize pollution associated with volatile organic compound (VOC) solvents through recycling.1
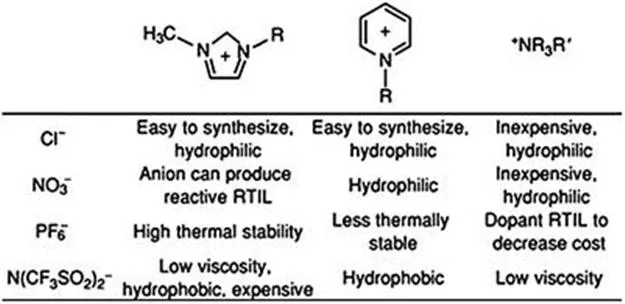
Some examples of common IL cations and anions and their general characteristics are shown in Figure 1.1. Imidazolium- and pyridinium-based ILs feature delocalized cations, whereas quaternary ammonium cations are asymmetrically substituted. A very wide range of properties, including viscosity, hydrophobicity or hydrophilicity, thermal and chemical stability, melting point, flammability and cost, can be tuned by judicious selection of the cation and anion.
Figure 1.1 Some common IL cations and anions and their properties.
Probably the first room temperature IL, ethylammonium nitrate (m.p. 12 °C), was reported by Walden2 in 1914. However, there was little interest in ILs until the mid-1970s when they attracted attention as electrolytes for batteries.3,4 Nowadays, ILs are being intensely investigated in a wide range of applications, including use as solvents in a wide range of chemical processes.5 ILs have been used as solvents for Diels–Alder reactions,6 hydrogenations,7 alkylations,8 Friedel–Crafts reactions,9 Heck reactions,10 Suzuki couplings,11,12 metathesis reactions,13,14 and many others.
To the best of our knowledge, the first polymerization in ILs was reported in 1990 by Carlin et al.15 They reported that TiCl4 and AlEthylCl2 in AlCl3/1-ethyl-3-methylimmidazolium chloride ([EMIM]Cl) could polymerize ethylene in low yields. Subsequent work by the same group, where TiCl4 was replaced by Cp2TiCl2, gave higher yields.16 This pioneering work, using ILs as a reaction medium for polymerization, has inspired numerous researchers over the past quarter of a century to investigate a wide range of different types of polymerization in ILs. While much of this work was inspired by the “green” aspects of ionic liquids (very low vapor pressure and potential for recycling), it quickly became apparent that chemistry could often proceed differently (faster polymerization rates, higher molecular weights, enhanced yields, etc.) in ILs. Providing a review of the field of polymerization in ionic liquids, with particular attention to developments over the past several years, is the subject of this chapter. The reader is referred to earlier reviews in this field for additional details on work in this area.17–23
1.2 ILs in Conventional Free Radical Polymerization
Free radical polymerization, because of its compatibility with a wide range of monomers having different types of functional groups, is one of the most widely used polymerization techniques. Free radical solution polymerization is of great commercial importance as dilution of radical polymerizations with a solvent, typically a VOC, is effective in controlling viscosity and the exotherm accompanying polymerization. Hong et al.24 noted large increases in the rate of polymerization and much higher molecular weights for free radical polymerization of methyl methacrylate (MMA) in [BMIM]PF6 as compared to polymerizations carried out under identical conditions in VOCs. These effects were attributed at least in part to the high viscosity of the polymerization medium. A “diffusion-controlled termination” mechanism was proposed to explain the decreased rate of chain termination in these viscous systems. A decrease in termination rate could explain a simultaneous increase in the rate of polymerization and molecular weight.24 In contrast to variations in the rate of polymerization and molecular weight, the polymers synthesized in RTILs have similar glass transition temperatures and microstructures as compared to those obtained in benzene or in bulk, based upon thermal analysis and 13C-NMR experiments.25 Since then, other groups26–28 have reported similar behavior, high molecular weights and rapid polymerization rates, for MMA and other methacrylates in [BMIM]PF6. Harrison et al.29,30 used pulse laser polymerization (PLP) techniques to polymerize MMA in [BMIM]PF6. They found that both the propagation and termination rates, kp and kt, respectively, were strongly affected by the presence of [BMIM]PF6. They attributed the increase in propagation rate to the high polarity of the ionic liquid solution, which reduces the activation energy of propagation via charge–transfer interactions. The termination rate decrease was attributed to the increased viscosity of the polymerization medium. Both the increase of kp and the decrease of kt combine to account for a ten-fold increase in the overall rate of polymerization. Subsequent PLP studies31,32 have reported kt to be decreased by an order of magnitude and kp to be increased by a factor of 4 for free radical polymerization in ILs.
Polenz and co-workers recently studied the polymerization of MMA co-initiated by imine bases and found that the reaction was accelerated greatly by even a trace amount of IL.33 They demonstrated that the polymerization proceeded via a free radical mechanism, with the addition of IL decreasing the activation energy of polymerization and increasing the rate of polymerization. These effects were attributed to interactions between the IL and the imine base. Cheng et al.34 studied free radical polymerization of acrylonitrile in [BMIM]BF4 using AIBN as an initiator. This team found that ionic liquids are excellent media for obtaining high molecular weight polymers. They attribute this finding to the low chain transfer constants for ILs and their ability to stabilize growing radical chain ends. Puttick et al.35 used NMR to investigate nanoscale domains formed in dialkylimidazolium ILs. When polymerizing MMA in this type of IL, it was shown that the reactants and intermediates have different affinities for nanodomains that form within the IL. Segregation of different species within these domains accounts for the unusually high polymerization rates and increased molecular weights.
Many early studies on free radical polymerization in ILs used ...