Perovskite Solar Cells: Principle, Materials And Devices
Principle, Materials and Devices
Eric Wei-Guang Diau, Peter Chao-Yu Chen;;;
- 244 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
Perovskite Solar Cells: Principle, Materials And Devices
Principle, Materials and Devices
Eric Wei-Guang Diau, Peter Chao-Yu Chen;;;
À propos de ce livre
-->
Energy and climate change are two of the most critical issues nowadays. These two topics are also correlated to each other. Fossil fuels are the main energy supplies that have been used in modern history since the industrial revolution. The impact of CO 2 emission has been a major concern for its effect on global warming and other consequences. In addition, fossil fuels are not unlimited. Due to the increasing demands for energy supplies, alternative renewable, sustainable, environmentally friendly energy resources are desirable.
Solar energy is an unlimited, clean, and renewable energy source, which can be considered to replace the energy supply of fossil fuel. The silicon solar cell is one of the dominant photovoltaic technologies currently, which converting sunlight directly into electric power with around 20% efficiency. This technique was been widely used in mainstream solar energy applications for decades, though the relatively energy-demanding production process remained with challenges to be resolved.
Recently, emerging photovoltaic technologies such as organometal halide hybrid perovskite solar cell has attracted tremendous attention due to their promising power conversion efficiencies (over 22%) and ease of fabrication. Their progress roadmap is unprecedented in photovoltaic history from the material development and efficiency advancement perspective. Beyond the rapid progress achieved in the last few years, it is expected that this novel technology would make an impact on the future solar cell market providing long-term stability and Pb content issues are addressed. These challenges rely on a better understanding of materials and device function principles. The scope of this book is to provide a collection on the recent investigations from fundamental process, materials development to device optimization for perovskite solar cells.
--> Contents:
- Additive-Assisted Controllable Growth of Perovskites (Yixin Zhao and Kai Zhu)
- Control of Film Morphology for High Performance Perovskite Solar Cells (Cheng-Min Tsai, Hau-Shiang Shiu, Hui-Ping Wu and Eric Wei-Guang Diau)
- Sensitization and Functions of Porous Titanium Dioxide Electrodes in Dye-Sensitized Solar Cells and Organolead Halide Perovskite Solar Cells (Seigo Ito)
- P-Type and Inorganic Hole Transporting Materials for Perovskite Solar Cells (Ming-Hsien Li, Yu-Hsien Chiang, Po-Shen Shen, Sean Sung-Yen Juang and Peter Chao-Yu Chen)
- Hole Conductor Free Organometal Halide Perovskite Solar Cells: Properties and Different Architectures (Sigalit Aharon and Lioz Etgar)
- Stability Issues of Inorganic/Organic Hybrid Lead Perovskite Solar Cells (Dan Li and Mingkui Wang)
- Time-Resolved Photoconductivity Measurements on Organometal Halide Perovskites (Eline M Hutter, Tom J Savenije and Carlito S Ponseca Jr)
-->
--> Readership: Graduate students and researchers in chemistry, materials science and photovoltaics. -->
Keywords:Perovskite Solar Cells;Hole Transporting Materials;Stability;THz SpectroscopyReview:0
Foire aux questions
Informations
| 1 | Additive-Assisted Controllable Growth of Perovskites |
| DSSC | dye-sensitized solar cell |
| EDX | energy-dispersive X-ray |
| FTO | fluorine-doped tin oxide |
| IPA | isopropanol |
| Jsc | short-circuit current density |
| MAI | methylammonium iodide |
| MAPbI3 | methylammonium lead iodide |
| PCE | power conversion efficiency |
| PSC | perovskite solar cells |
| PV | photovoltaic |
| SEM | scanning electron microscopy |
| TGA | thermogravimetric analysis |
| TGA-MS | thermogravimetric analysis-mass spectroscopy |
| Voc | open circuit voltage |
| XPS | X-ray photoelectron spectra |
| XRD | X-ray diffraction |
I. Introduction
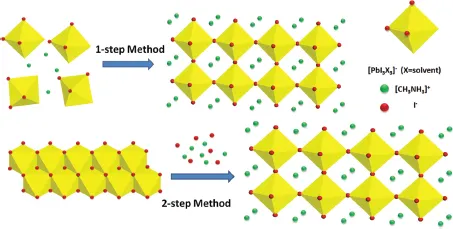
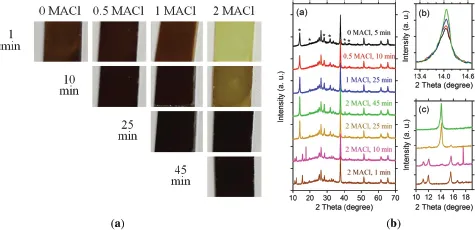
II. Cl-Based Additives
A.Role of MACl in One-Step Solution Processing