
eBook - ePub
Chromatography
Principles and Instrumentation
Mark F. Vitha
This is a test
Partager le livre
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Chromatography
Principles and Instrumentation
Mark F. Vitha
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Provides students and practitioners with a solid grounding in the theory of chromatography, important considerations in its application, and modern instrumentation.
- Highlights the primary variables that practitioners can manipulate, and how those variables influence chromatographic separations
- Includes multiple figures that illustrate the application of these methods to actual, complex chemical samples
- Problems are embedded throughout the chapters as well as at the end of each chapter so that students can check their understanding before continuing on to new sections
- Each section includes numerous headings and subheadings, making it easy for faculty and students to refer to and use the information within each chapter selectively
- The focused, concise nature makes it useful for a modular approach to analytical chemistry courses
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Chromatography est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Chromatography par Mark F. Vitha en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Ciencias físicas et Química analítica. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
CHAPTER 1
Many “real-world” samples are mixtures of dozens, hundreds, or thousands of chemicals. For example, medication, gasoline, blood, cosmetics, and food products are all complex mixtures. Common analyses of such samples include quantifying the levels of drugs – both legal and illegal – in blood, identifying the components of gasoline as part of an arson investigation, and measuring pesticide levels in food.
FUNDAMENTALS OF CHROMATOGRAPHY
Chromatography is a technique that separates the individual components in a complex mixture. Fundamental intermolecular interactions such as dispersion, hydrogen bonding, and dipole–dipole forces govern the separations. Once separated, the solutes can also be identified and quantified. Because of its ability to separate, quantify, and identify components, chromatography is one of the most important instrumental methods of analysis, both in terms of the number of instruments worldwide and the number of analyses conducted every day.
1.1 THEORY
Chromatography separates components in a sample by introducing a small volume of the sample at the start, or head, of a column. A mobile phase, either gas or liquid, is also introduced at the head of the column. When the mobile phase is a gas, the technique is referred to as gas chromatography (GC) and when it is a liquid, the technique is called liquid chromatography (LC). Unlike the sample, which is injected as a discrete volume, the mobile phase flows continuously through the column. It serves to push the molecules in the sample through the column so that they emerge, or “elute” from the other end.
Two particular modes of LC and GC, known as reversed-phase liquid chromatography (RPLC) and capillary gas chromatography, account for approximately 85% of all chromatographic analyses performed each day. Therefore, we focus on these two techniques here and leave discussions of specific variations to the chapters that describe LC and GC in greater detail.
In GC, the mobile phase, which is typically He, N2, or H2 gas, is delivered from a high-pressure gas tank. The gas flows through the column toward the low-pressure end. The column contains a stationary phase. In capillary GC, the stationary phase is typically a polymer film that is 0.25–5 µm thick (see Figure 1.1a). It is coated on the interior walls of a fused silica capillary column with an inner diameter of approximately 0.5 mm or smaller. The column is usually 10–60 m (30–180 ft) long.
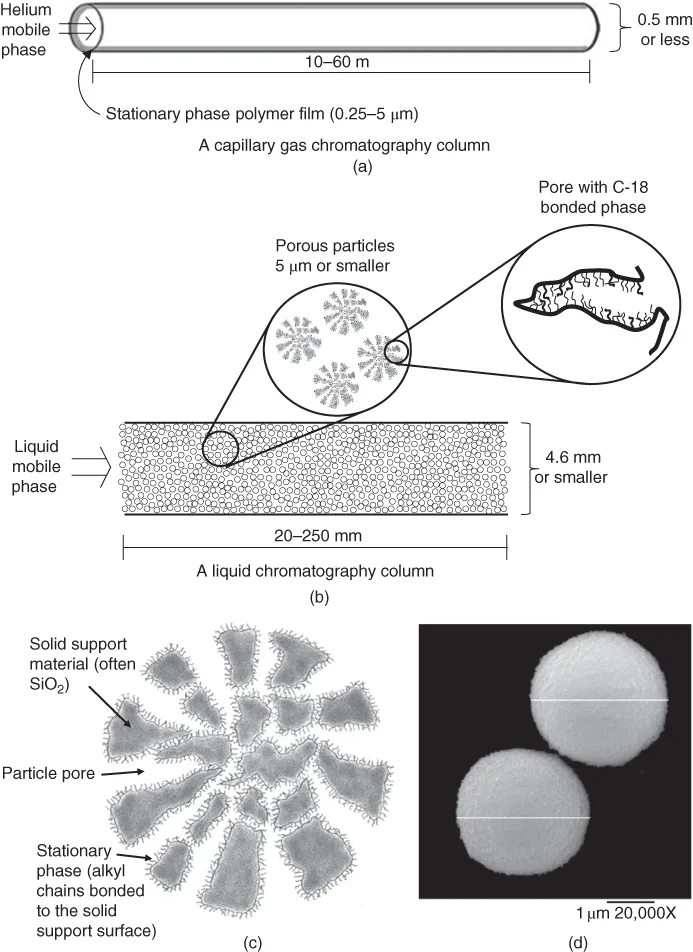
Figure 1.1 Representations of typical capillary gas (a) and liquid (b) chromatography columns. Figure (c) is a depiction of a cross section of a porous particle (shaded areas represent the solid support particles, white areas are the pores, and the squiggles on the surface are bonded alkyl chains. Figure (d) is an scanning electron microscope (SEM) image of actual 3 µm liquid chromatography porous particles. Note that the lines across the particle diameters have been added to the image and are not actually part of particles. (Source: Alon McCormick and Peter Carr. Reproduced with permission of U of MN.). It is worth taking time to note the different dimensions involved. For the GC columns, they range from microns (10−6 m) for the thickness of the stationary phase, to millimeters (10−3 m) for the column diameter, up to tens of meters for the column length. Note also that LC columns are typically much shorter than GC columns (centimeter versus meter).
RPLC is the most common mode of liquid chromatography. In RPLC, the mobile phase is a solvent mixture such as water with acetonitrile (CH3CN) that is forced through the column using high-pressure pumps. The column is typically made of stainless steel, has an inner diameter of 4.6 mm or smaller, and is only 20–250 mm (1–10 in.) in length (see Figure 1.1b). However, unlike most GC columns, most LC columns are packed with tiny spherical particles approximately 5 µm in diameter or smaller, as shown in Figure 1.1c and d. When rubbed between your fingers, the particles feel like talc or other fine powders. The particles are not completely solid, but rather are highly porous, with thousands of pores in each particle. The pores create cavities akin to caves within the particle....