
eBook - ePub
Atmospheric Aerosols
Life Cycles and Effects on Air Quality and Climate
Claudio Tomasi, Sandro Fuzzi, Alexander Kokhanovsky, Claudio Tomasi, Sandro Fuzzi, Alexander Kokhanovsky
This is a test
Partager le livre
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Atmospheric Aerosols
Life Cycles and Effects on Air Quality and Climate
Claudio Tomasi, Sandro Fuzzi, Alexander Kokhanovsky, Claudio Tomasi, Sandro Fuzzi, Alexander Kokhanovsky
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
The book describes the morphological, physical and chemical properties of aerosols from various natural and anthropogenic sources to help the reader better understand the direct role of aerosol particles in scattering and absorbing short- and long-wave radiation.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Atmospheric Aerosols est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Atmospheric Aerosols par Claudio Tomasi, Sandro Fuzzi, Alexander Kokhanovsky, Claudio Tomasi, Sandro Fuzzi, Alexander Kokhanovsky en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Physical Sciences et Atomic & Molecular Physics. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Chapter 1
Primary and Secondary Sources of Atmospheric Aerosol
Claudio Tomasi and Angelo Lupi
1.1 Introduction
Atmospheric aerosols are suspensions of any substance existing in the solid and/or liquid phase in the atmosphere (except pure water) under normal conditions and having a minimum stability in air assuring an atmospheric lifetime of at least 1 h. Generated by natural sources (i.e., wind-borne dust, sea spray, volcanic debris, biogenic aerosol) and/or anthropogenic activities (i.e., sulfates and nitrates from industrial emissions, wind-forced mineral dust mobilized in areas exploited for agricultural activities, fossil fuel combustion, and waste and biomass burning), aerosol particles range in size from a few nanometers to several tens of microns. As a result of internal cohesive forces and their negligible terminal fall speeds, aerosol particles can first assume sizes appreciably larger than the most common air molecules and subsequently increase to reach sizes ranging most frequently from less than 10−3 to no more than 100 µm (Heintzenberg, 1994). Particles with sizes smaller than 20–30 Å (1 Å = 10−10 m) are usually classified as clusters or small ions, while mineral and tropospheric volcanic dust particles with sizes greater than a few hundred microns are not considered to belong to the coarse aerosol class, since they have very short lifetimes. Aerosol particles grown by condensation to become cloud droplets are not classified as aerosols, although a cloud droplet needs a relatively small aerosol particle acting as a condensation nucleus for its formation under normal atmospheric conditions. Similarly, precipitation elements such as rain droplets, snowflakes, and ice crystals are not classified as aerosols (Heintzenberg, 1994). Although present in considerably lower concentrations than those of the main air molecules, aerosol particles play a very important role in numerous meteorological, physical, and chemical processes occurring in the atmosphere, such as the electrical conductivity of air, condensation of water vapor on small nuclei and subsequent formation of fog and cloud droplets, acid rains, scattering, and absorption of both incoming solar (shortwave) radiation and thermal terrestrial (longwave) radiation. The interaction processes between atmospheric aerosols and the downwelling and upwelling radiation fluxes of solar and terrestrial radiation at the surface play a major role in defining the radiation budget of our planet and, hence, the Earth's climate (Chylek and Coakley, 1974).
To give an idea of the shape of an aerosol particle suspended in dry air, a schematic representation of a particle originating from the aggregation of various kinds of particulate matter fragments is shown in Figure 1.1. It consists of several small unit structures of different chemical composition and origin (soluble acid substances, sodium chloride crystals of marine origin, ammonium sulfates, insoluble carbonaceous matter, insoluble mineral dust, and insoluble organic substances), held together by interparticle adhesive forces in such a way that an aerosol particle behaves as a single unit in suspension. Thus, the same particle often contains distinct homogeneous entities, which are internally mixed to form aggregates of different components.
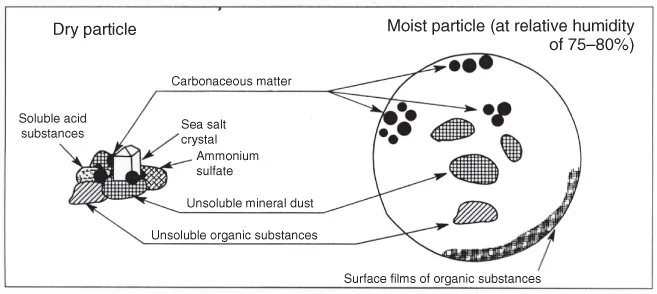
Figure 1.1 Schematic representation of an aerosol particle for dry air conditions (left) and humid air (for relative humidity (RH) = 75–80%) conditions (right), consisting of particulate matter pieces of soluble (i.e., soluble acid substances, sea-salt crystal, ammonium sulfates) and insoluble substances (carbonaceous matter, mineral dust, organic substances), which remain suspended inside the moist particle gradually growing by condensation until becoming a water droplet with soluble salts, acids, and organic compounds. (Adapted from a draft presented by Gottfried Hänel in a seminar given in 1985 at the FISBAT-CNR Institute, Bologna, Italy.)
The insoluble carbonaceous and organic substances often consist of gas-borne particulate matter pieces from incomplete combustion, which predominantly contain carbon and other combustion-produced materials. When the surrounding air relative humidity (RH) increases to reach values higher than 65–70%, the same particle (containing soluble substances) grows gradually by condensation of water vapor to become a water droplet in which pieces of insoluble matter are suspended, as can be seen in the (b) of Figure 1.1 (see also Hänel, 1976), while the various soluble materials reach different solution states as a result of their appreciably differing deliquescence properties. In this way, an internally mixed particle evolves assuming the characteristics of an aggregate consisting of different particulate phases. Figure 1.1 also shows that dry aerosol particles can often exhibit irregular shapes, which can considerably differ from the spherical one. Thus, the size of each real aerosol particle is generally evaluated in terms of an “equivalent” diameter a, for which the volume of such an ideal spherical particle is equal to that of the real particle.
Aerosol particles cover a size range of more than five orders of magnitude, with “equivalent” sizes ranging from 5 × 10−3 to 2.5 µm for fine particles and greater than 2.5 µm for coarse particles (Hinds, 1999). The fine particles include both (i) the so-called Aitken nuclei, having sizes mainly ranging from 5 × 10−3 to 5 × 10−2 µm, and (ii) the so-called “accumulation” particles having sizes ranging from 5 × 10−2 to about 2 µm. In this classification, it is worth mentioning that (i) the nuclei constitute the most important part of the so-called ultrafine particles (which have sizes <10−1 µm) and mainly form through condensation of hot vapors during combustion processes and/or nucleation of atmospheric gaseous species to form fresh particles and (ii) the accumulation particles are mainly generated through coagulation of small particles belonging to the nuclei class and condensation of vapors onto existing particles, inducing them to grow appreciably. Consequently, the particle concentration within this size subrange increases, and the accumulation mode becomes gradually more evident, so named because the particle removal mechanisms are poorly efficient in limiting the concentration of such an intermediate-size class of particles. Therefore, such particles have longer residence times than the nuclei, and ...