Biological Sciences
Polysaccharides
Polysaccharides are complex carbohydrates made up of multiple sugar units linked together. They serve as energy storage molecules in organisms and play structural roles in cell walls and extracellular matrices. Examples of polysaccharides include starch, glycogen, and cellulose, each with distinct functions and properties in biological systems.
Written by Perlego with AI-assistance
Related key terms
1 of 5
11 Key excerpts on "Polysaccharides"
- eBook - ePub
- Donald Voet, Judith G. Voet(Authors)
- 2021(Publication Date)
- Wiley(Publisher)
10 Sugars and Polysaccharides1 MonosaccharidesA. ClassificationB. Configurations and ConformationsC. Sugar Derivatives2 PolysaccharidesA. Carbohydrate AnalysisB. DisaccharidesC. Structural Polysaccharides: Cellulose and ChitinD. Storage Polysaccharides: Starch and GlycogenE. Glycosaminoglycans3 GlycoproteinsA. ProteoglycansB. Bacterial Cell WallsC. Glycoprotein Structure and FunctionD. GlycomicsCarbohydrates or saccharides (Greek: sakcharon, sugar) are essential components of all living organisms and are, in fact, the most abundant class of biological molecules. The name carbohydrate, which literally means “carbon hydrate,” stems from their chemical composition, which is roughly (C ⋅ H2 O)n, where n ≥ 3. The basic units of carbohydrates are known as monosaccharides. Many of these compounds are synthesized from simpler substances in a process named gluconeogenesis (Section 15-7 ). Others (and ultimately nearly all biological molecules) are the products of photosynthesis (Section 21-3 ), the light-powered combination of CO2 and H2 O through which plants and certain bacteria form “carbon hydrates.” The metabolic breakdown of monosaccharides (Chapters 15 and 19) provides much of the energy used to power biological processes. Monosaccharides are also principal components of nucleic acids (Section 4-1A ), as well as important elements of complex lipids (Section 11-1D ).Oligosaccharides consist of a few covalently linked monosaccharide units. They are often associated with proteins (glycoproteins) and lipids (glycolipids) in which they have both structural and regulatory functions (glycoproteins and glycolipids are collectively called glycoconjugates). Polysaccharides consist of many covalently linked monosaccharide units and have molecular masses ranging well into the millions of daltons. They have indispensable structural functions in all types of organisms but are most conspicuous in plants because cellulose, their principal structural material, comprises up to 80% of their dry weight. Polysaccharides such as starch in plants and glycogen - eBook - PDF
Polysaccharide Dispersions
Chemistry and Technology in Food
- Reginald H. Walter, Steve Taylor(Authors)
- 1997(Publication Date)
- Academic Press(Publisher)
CHAPTER I Origin and Characteristics of Polysaccharides I. Introduction Polysaccharides are a class of biopolymers constituted with simple sugar monomers. Those used in commerce and industry are isolates from terres- trial and marine plants or are principally the exogenous metabolites of some bacteria; many are modified by partial organic synthesis, and a few are the product of total biochemical synthesis. Isolates from the same species, but from different culfivars, are remarkably chemically uniform (Jones and Smith, 1949). These so-called natural gums and mucilages have teleological significance in plant metabolism and function; one primary responsibility attributed to many of them is winter and drought hardinessma consequence of their water-binding characteristics. When extracted and purified, they are a major food item that is universally recognized as safe for human consump- tion. They are additionally an important industrial, scientific, and medical commodity. In petroleum recovery, mixtures of Polysaccharides and sand are pumped into oil-well crevices to provide transport channels for oil and gas. In science, Polysaccharides are crosslinked for improved mechanical strength and acid, heat, and shear resistance, for use as adsorbents and ion exchang- ers. Glycotechnology is a currently active area of pharmaceutical and medi- cal research on oligosaccharides for drugs. Cellulose, the most abundant polysaccharide, is the structural compo- nent of plant tissues; starch is the energy compound stored predominantly in seeds and tubers; glycogen is the animal counterpart of starch, but with shorter, more numerous branches. Cellulose and starch cohabit plant tissues with hemicellulose, protoplasm, lipid, and mineral matter in an organization interrupted by intercellular spaces that can amount to more than 50% of the total volume of some fruits and vegetables. A number of useful polysaccha- rides and their origins are listed in Table I. - eBook - ePub
Pullulan
Processing, Properties, and Applications
- Shakeel Ahmed, Aisverya Soundararajan, Shakeel Ahmed, Aisverya Soundararajan(Authors)
- 2020(Publication Date)
- Jenny Stanford Publishing(Publisher)
Polysaccharides, commonly known as “Cinderella of biopolymers,” are molecules composed of monosaccharide residues, which are joined together by O-glycosidic linkages. Naturally, Polysaccharides are produced by various biological entities such as plants, microbes, seaweeds, and animals. Polysaccharides were often found to have linear structure, and it may also contain various degrees of branching. Despite their structure, they also vary in various components such as monosaccharide composition, linkage type, pattern of linkage, chain type, degree of polymerization, etc. based on the source of production. This variable structure of Polysaccharides is responsible for their physiochemical and biological characteristics, which in turn is responsible for their diversified applications. They are used in various industries such as pharmaceutical industry, food industry, and other non-food industries. With further advancements in technologies, Polysaccharides have been structurally modified and studied for their various functions. In addition to these, the formation of nanocomposite with these polymers has been studied extensively for various applications from biosensors to drug delivery They also find their importance in environmental applications by taking up several xenobiotics. Therefore, in this chapter, various Polysaccharides from nature have been discussed in detail along with their applications.1.1IntroductionPolysaccharides are carbohydrate molecules made up of monosaccharide units bonded together by glycosidic linkages with a molecular weight of hundreds to thousands of Daltons [112 , 149 ]. Depending on the number of monosaccharide units, they have been classified as linear or branched Polysaccharides. They also have several functional groups such as hydroxyl group, carboxylic acid group, and amino group [103 ]. The naturally existing Polysaccharides exhibit distinct features in terms of molecular weight, monosaccharide composition, glycosidic linkage types and patterns, configuration, charging properties, chain shapes, degree of polymerization, degree of branching, etc. These diversified structural properties of Polysaccharides determine their functional properties such as solubility, flow behavior, gelling potential, surface and interfacial properties [54 , 70 ].Polysaccharides are the integral component of plant biomass, and 90% of the total polysaccharide produced on earth is constituted by vegetables. Apart from vegetables, they are produced by bacteria, fungi, algae, and animals [39 , 99 ]. Different types of Polysaccharides include starch, glycogen, cellulose, pectin, chitin, hemicellulose, lignin, etc [35 ]. Since ancient times, Polysaccharides have been used in several industrial applications such as pharmaceuticals, food and nutrition, and other non-food industries. In the food industry, it has been used as emulsifiers, stabilizer, thickening agent, and as packaging materials [90 , 104 ]. In addition to these, they play a vital role in the pharmaceutical industry due to their several advantageous characteristics such as biocompatibility, biodegradability, and ability of chemical modification [151 ]. They have been widely applied as binders, drug-release modifiers, thickeners, stabilizers, disintegrants, suspending agents, gelling agents, and as biodhesives [53 - eBook - ePub
- Dr Susan Brooks, Dr M Dwek, Udo Schumacher(Authors)
- 2023(Publication Date)
- Garland Science(Publisher)
3 Polysaccharides3.1 Introduction – how are Polysaccharides defined?
Polysaccharides are polymers of monosaccharide units linked by glycosidic bonds into linear or branching chains. Branching is possible because of the diversity in glycosidic bonds that can be formed to link any of the hydroxyl groups of the component monosaccharides (Chapter 1 ). The two main patterns of branching form either comb- or tree-like structures (Figure 3.1 ). There is no rigorously defined division between oligosaccharides and Polysaccharides (see 1.16.3 ), but Polysaccharides are usually accepted to contain more than 10–20 monosaccharide residues, and in practice are usually much larger than this, typically comprising ≈ 100 monosaccharide units or more, with cellulose comprising ≈ 3000 monosaccharide units. Connective tissue Polysaccharides which contain one or more amino sugar moieties are usually referred to as glycosaminoglycans, and are considered separately in Chapter 8 - eBook - PDF
- Jose Perez-Castineira(Author)
- 2020(Publication Date)
- De Gruyter(Publisher)
– Sugar: Many monosaccharides and disaccharides are sweet, hence their trivial name sugars, although table sugar is only composed of sucrose, a disaccha- ride. Carbohydrates are also known as glycids (from the Greek glykys, glykeros: sweet) or saccharides (from the latin saccharum: sugar). – Polysaccharides: Polymers composed of more than 12 (or 20) residues of mono- saccharides. Polysaccharides are named as homo- or heteroPolysaccharides depending on whether they are composed of a single type or more than one type of monosaccharide, respectively. Oligo- and Polysaccharides can be linear or branched and they may undergo further chemical modifications (see below). – Glycan: Although this term is considered a synonym of polysaccharide by the IUPAC [2], biochemists use it for those oligo- or Polysaccharides that bind to other biomolecules. – Glycoconjugates: Biochemical compounds consisting of a glycan moiety linked to proteins or lipids. The process by which a glycan is attached to another bio- molecule is known as glycosylation when it is catalyzed by an enzyme, or https://doi.org/10.1515/9783110595482-003 glycation when it is just a chemical non-mediated process. There are several types of glycoconjugates: – Glycoproteins/glycopeptides: Proteins/peptides (Chapter 5) containing oligosaccharide chains covalently attached to amino acid side chains. – Peptidoglycans: Generally composed of linear heteroPolysaccharides cova- lently linked with short peptides. – Glycolipids: Lipids (usually 1,2-diacylglycerols, see Chapter 4) with a monosaccharide or oligosaccharide linked. – LipoPolysaccharides: Complex glycolipids that occur in the outer mem- brane of Gram-negative bacteria. They induce immune response in many species, including humans. - eBook - PDF
Sweeteners
Nutritional Aspects, Applications, and Production Technology
- Theodoros Varzakas, Athanasios Labropoulos, Stylianos Anestis(Authors)
- 2012(Publication Date)
- CRC Press(Publisher)
2.5 Polysaccharides Polysaccharides are very important for human nutrition and widely distributed in nature. It is estimated that more than 90% of the considerable carbohydrate mass in nature is in the form of poly-saccharides. Examples include storage Polysaccharides, such as starch and glycogen, and structural Polysaccharides, such as cellulose and chitin. Polysaccharides (glycans) are high molecular weight polymers of monosaccharides and are named after their component monosaccharide. The degree of polymerization (DP), which is determined by the number of monosaccharide units in a chain, varies from a hundred to a few hundred thousand. Polysaccharides can be either linear or branched. Based on the number of different monomers present, Polysaccharides can be divided into homopolysac-charides, consisting of only one kind of monosaccharide (e.g., cellulose and starch amylose, which are linear and starch amylopectin, which is branched), and heteroPolysaccharides, consisting of two or more kinds of monosaccharide units (e.g., hemicellulose and pectins). Hydrolysis of glycosidic bonds joining monosaccharide (glycosyl) units in Polysaccharides can be catalyzed by either acids or enzymes (BeMiller and Whistler 1996; Belitz et al. 2009). Although it seems macroscopically and microscopically amorphous, X-ray analysis has revealed Polysaccharides’ microcrystalline structure. Depending on the structure, Polysaccharides may have distinct properties from their monosaccharide building blocks. Thus, they do not have sweet taste, they do not reduce Fehling’s solution, and they differ in solubility—they may be easily soluble (glycogen) or form colloidal solutions in water (starch) or are insoluble in warm water (cellulose). 2.5.1 HomoPolysaccharides The major homoPolysaccharides are starch, cellulose, and glycogen. 2.5.1.1 Starch Among food carbohydrates, starch occupies a unique position. - eBook - PDF
- Allan Blackman, Steven E. Bottle, Siegbert Schmid, Mauro Mocerino, Uta Wille(Authors)
- 2022(Publication Date)
- Wiley(Publisher)
CHAPTER 22 Carbohydrates LEARNING OBJECTIVES After studying this chapter, you should be able to: 22.1 define carbohydrates 22.2 describe monosaccharides using aldose/ketose terminology 22.3 understand and describe the cyclic structure of monosaccharides 22.4 describe the chemical reactions of monosaccharides 22.5 explain disaccharides and oligosaccharides 22.6 define Polysaccharides and describe starch, glycogen and cellulose. Carbohydrates is probably the chemical term that is most widely used by the general public. Commonly referred to as ‘carbs’, it seems everyone has an idea of how much, or how little, or what type we should be consuming in our diets. Carbohydrates are in fact a major class of organic molecules that are important not only in food, but more broadly in biochemistry, medicines, agriculture and even as structural materials. Carbohydrates act as storehouses of chemical energy (glucose, starch, glycogen) and are components of supportive structures in plants (cellulose), crustacean shells (chitin) and connective tissues in animals (Polysaccharides). Carbohydrates are also essential components in the nucleic acids RNA(d-ribose) and DNA (2-deoxy- d-ribose), and they play crucial roles in cell surface and membrane recognition that are necessary for cell function. Small carbohydrate molecules, such as glucose, are readily soluble in water and so can be transported through the vascular system to meet a plant’s or animal’s energy requirements. Chemists are increasingly interested in carbohydrates as a potential solution for many of the problems caused by the burning of fossil fuels for energy. Increasing research efforts are being focused on ‘biofuels’, largely ethanol, derived from cellulose. Cellulose accounts for approximately three-quarters of the dry weight of the plant, where it is used to provide plant cell walls with strength and rigidity. - eBook - PDF
Biotemplating: Complex Structures From Natural Materials
Complex Structures from Natural Materials
- Simon Robert Hall(Author)
- 2009(Publication Date)
- ICP(Publisher)
28 Chapter 3 Complex Polysaccharides In the last chapter, we considered the simpler sugars as potential candidates for biotemplating. These molecules are limited however, owing to their relative structural simplicity and act in a more passive role in the templating of materials. With Polysaccharides, the potential for more complex templating emerges as they are now able to crystallize with long-range order and thereby impart this complexity to the final material. With increasing chain length, there exists the possibility to synthesize an incredibly large number of Polysaccharides, both in the laboratory and in nature. A flavour of the range of these will be given, but the majority of the chapter will be devoted to the most commonly researched complex Polysaccharides. 3.1 Structure and properties As the number of sugar residues increases and the glycosidic bonds multiply, longer and longer chains form. With the potential for glycosidic bonds to appear which branch the chain structure, the number of structurally distinct Polysaccharides is almost limitless. With increasing chain length, the physical properties alter progressively away from the sweet-tasting, soluble simple monosaccharides to molecules which tend to be insoluble, tasteless and in the case of higher molecular weight Polysaccharides, form viscous, sticky masses. Polysaccharides have a general formula of C n (H 2 O) n-1 where n is usually a large number between 200 and 2500, although some of the larger Polysaccharides have molecular weights in the hundreds of thousands of daltons. Despite the large range of molecular weights and structures, the Polysaccharides can be distinguished broadly by the type of glycosidic bond present in the molecule. In making a glycosidic bond, there are two possibilities for linking the monosaccharide units together. The first is the alpha-linkage where (for a D-sugar configuration) the bond is made at the anomeric carbon of one - eBook - ePub
Annual Plant Reviews, Plant Polysaccharides
Biosynthesis and Bioengineering
- Peter Ulvskov(Author)
- 2010(Publication Date)
- Wiley-Blackwell(Publisher)
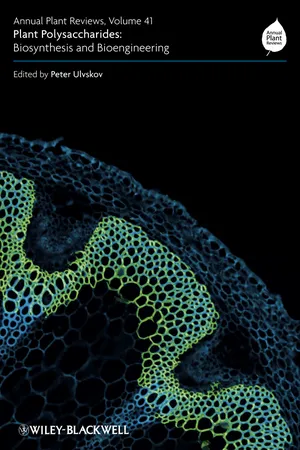
Chapter 14 PLANT CELL WALL BIOLOGY: Polysaccharides IN ARCHITECTURAL AND DEVELOPMENTAL CONTEXTS14.1 IntroductionMaureen C. McCann1 and J. Paul Knox21 Biological Sciences, Purdue University, West Lafayette, IN 47907, USA2 Centre for Plant Sciences, Biological Sciences, University of Leeds, Leeds LS2 9JT, UKAbstract:Land plant cell walls contain in the region of 11 major Polysaccharides that are grouped as cellulose, hemicelluloses and pectins. Methodologies involving the use of molecular probes and advanced spectroscopies are revealing considerable diversity and complexity as to how these Polysaccharides are configured within cell wall structures. Our knowledge of the integration of selected sets of Polysaccharides into diverse and multifunctional primary and secondary cell walls is currently increasing although many challenges remain. In addition to understanding the structure and function of Polysaccharides in the architectures of individual cell walls and in varied developmental contexts there is also a taxonomic dimension to cell wall Polysaccharides that is likely to reveal insights into functions. Challenges for future studies include the understanding of aspects of possible polysaccharide redundancy during cell wall assembly and function, and of how specific Polysaccharides are integrated with the mechanical attributes of cell walls and the placing of specific polysaccharide dynamics within physiological frameworks of regulation.Keywords: architecture; carbohydrate-binding modules; cell wall biology; microstructure; monoclonal antibodies; plant cell adhesion; spectroscopy.Cell walls are central to many cell processes that are fundamental to plant growth and survival. Cell walls are also significant materials in the contexts of food, paper, polymers, textiles and fuel. Many of the sets of plant Polysaccharides discussed elsewhere in this book are quantitatively the most important macromolecular components of cell walls. Our understanding of how these sets of Polysaccharides are assembled to provide functionally diverse, mechanically flexible but robust sets of cell walls is the basis of this chapter. Cell wall Polysaccharides are currently separated into cellulose, hemicelluloses and pectic polymers – groupings largely derived from both structural features and solubilization properties. The latter two groups contain polysaccharides of considerable structural diversity and complexity and, in addition, contain polymers that are subject to structural modulation within cell walls. - eBook - PDF
- Stephen G Rees-Jones(Author)
- 1987(Publication Date)
- Elsevier Science(Publisher)
6 Carbohydrates: sugars and Polysaccharides Wood, paper, plant fibres, and the water-soluble plant gums used as adhesives and binding media are all made up of compounds coming within this category. Originally the term carbohydrates was given to compounds of general empirical formula C x (H 2 0) y since they were thought of as 'hydrates of carbon'. This idea was soon dropped and the expression became less restrictive as to the ratios of the three elements, but many simple compounds made up of carbon, hydrogen, and oxygen are still excluded. In practice, carbohydrates can be taken to refer to the sugars (monosaccharides) and their polymers — the Polysaccharides. These are probably the most abundant organic materials on the earth's surface since, in the form of cellulose and starch, they form a good proportion of the bulk of trees and plants. As is well known they are built up from water and the carbon dioxide in the atmosphere by the process of photosynthesis ; the light energy from the sun being utilized for the purpose through the intermediacy of the green plant pigment, chlorophyll. 6.1 Monosaccharides Carbohydrate chemistry is a vast subject and difficult to condense to a brief summary. The sugars themselves are quite complex, they can commonly exist in several different chemical forms and they combine with one another in different possible ways. This will be illustrated by reference to one of the most common sugars, glucose. Glucose has molecular formula C 6 H 1 2 0 6 . It has five hydroxyl groups and a carbonyl group and, since this latter can be oxidized to an acid, it must be an aldehyde rather than a ketone. Glucose can be converted to hexanoic acid and so the carbons must be present as a straight chain. These features are accommodated by the following structure: CHO I CHOH I CHOH I CHOH I CHOH I CH„OH This, however, is not the end of the matter. - Romano Piras(Author)
- 2012(Publication Date)
- Academic Press(Publisher)
SESSION III FUNCTION AND ORGANIZATION OF CARBOHYDRATE CONTAINING POLYMERS SESSION CHAIRMAN: W. T. J. MORGAN This page intentionally left blank BIOSYNTHESIS OF Polysaccharides FROM SUGAR NUCLEOTIDES IN PLANTS W. Z. Hassid Department of Biochemistry University of California Berkeley, California 94720 Many of the carbohydrate metabolic processes in plants are similar to those which occur in animals and micro-organisms. However, various processes take place in plants through different pathways. There are also many of the carbohydrate compounds that are commonly present in plants which are not found in animals or microorganisms. While sucrose, raffinose, cellulose, pectin, starch, numerous glycosides, and the monosaccharides, IMnannose, D^xylose, and L^arabinose (primarily as polymers) are practically always present in higher plants, they are not found in animals. Some of these, namely, sucrose, cellulose, pectin and raffinose are seldom present in microorganisms. Biosynthesis of complex saccharides from various substrates It is now generally accepted that most of the complex carbohydrates in nature are synthesized via sugar nucleo-tide intermediates. A limited number of enzymes are known to utilize ct-I>-glucose 1-phosphate as substrate, leading to the formation of complex sugars. These are: glycogen phosphorvlase (1), starch phosphorvlase (2), sucrose phos-phorylase (3), maltose phosphorvlase (A), cellobiose phos-phorylase (5) and laminaribiose phosphorylase (6). Their formation, however, does not appear to be a normal physio-logical process; these enzymes appear to act only in a de-gradative capacity. The number of enzymes that lead to the formation of Polysaccharides (primarily bacterial poly-saccharides) from disaccharides is also limited. The two best known enzymes that form Polysaccharides from sucrose 315
Index pages curate the most relevant extracts from our library of academic textbooks. They’ve been created using an in-house natural language model (NLM), each adding context and meaning to key research topics.