
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Nuclear magnetic resonance (NMR) spectroscopy is one of the most powerful and widely used techniques in chemical research for investigating structures and dynamics of molecules. Advanced methods can even be utilized for structure determinations of biopolymers, for example proteins or nucleic acids. NMR is also used in medicine for magnetic resonance imaging (MRI). The method is based on spectral lines of different atomic nuclei that are excited when a strong magnetic field and a radiofrequency transmitter are applied. The method is very sensitive to the features of molecular structure because also the neighboring atoms influence the signals from individual nuclei and this is
important for determining the 3D-structure of molecules.
This new edition of the popular classic has a clear style and a highly practical, mostly non-mathematical approach. Many examples are taken from organic and organometallic chemistry, making this book an invaluable guide to undergraduate and graduate students of organic chemistry, biochemistry, spectroscopy or physical chemistry, and to researchers using this well-established and extremely important technique. Problems and solutions are included.
important for determining the 3D-structure of molecules.
This new edition of the popular classic has a clear style and a highly practical, mostly non-mathematical approach. Many examples are taken from organic and organometallic chemistry, making this book an invaluable guide to undergraduate and graduate students of organic chemistry, biochemistry, spectroscopy or physical chemistry, and to researchers using this well-established and extremely important technique. Problems and solutions are included.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Introduction
Of the important spectroscopic aids that are at the disposal of the chemist for use in structure elucidation, nuclear magnetic resonance (NMR) spectroscopy is one of the major tools. When, in December 1945 and in January 1946, two groups of physicists in the United States working independently – Edward M. Purcell, Howard C. Torrey, and Richard V. Pound at Harvard University on the US east coast and Felix Bloch, William W. Hansen, and Martin Packard at Stanford University in California – first succeeded in observing the phenomenon of NMR in solids and liquids they set the starting point for the unforeseen development of a new branch of science. The impact of their discovery was soon recognized and Bloch and Purcell received the Nobel Prize in Physics in 1952 (Figures 1.1 and 1.2).
Figure 1.1 The founding fathers of nuclear magnetic resonance: Felix Bloch (1905–1983) (a) (Reprinted with permission from Reference [1]. Copyright 1985 International Society of Magnetic Resonance.) and Edward M. Purcell (1912–1997) (b). Courtesy of Physics Department, Harvard University.
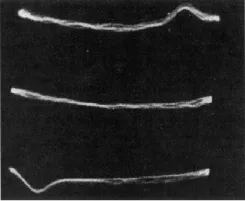
Figure 1.2 The first proton NMR signal from a water sample as seen on the screen of an oscilloscope by Bloch, Hansen, and Packard at Stanford University, California, USA, in January 1946 (Reprinted with permission from [2]. Copyright 1946 by the American Physical Society).
At the beginning of the 1950s, the phenomenon was called upon for the first time in the solution of a chemical problem. Since then its importance has steadily increased – a situation highlighted by three additional Nobel Prizes: in 1991 to Richard R. Ernst from the Eidgenössische Technische Hochschule (ETH) Zürich, Switzerland, for his outstanding contributions to the development of experimental NMR techniques, in 2002 to Kurt Wüthrich from the same institution for his contributions to structural biology, and in 2003 to Paul C. Lauterbur from the University of Illinois at Urbana-Champaign, and Sir Peter Mansfield, University of Nottingham, UK, for the invention of NMR imaging, known today as magnetic resonance imaging (MRI).
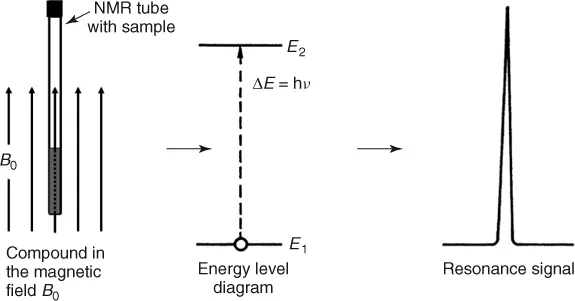
The physical foundation of NMR spectroscopy lies in the magnetic properties of atomic nuclei. The interaction of the nuclear magnetic moment with an external magnetic field, B0, leads, according to the rules of quantum mechanics, to a nuclear energy level diagram, because the magnetic energy of the nucleus is restricted to certain discrete values Ei, the so-called eigenvalues. Associated with the eigenvalues are the eigenstates, which are the only states in which an elementary particle can exist. They are also called stationary states. Through a radiofrequency (RF) transmitter, transitions between these states can be stimulated. The absorption of energy is then detected in an RF receiver and recorded as a spectral line, the so-called resonance signal (Figure 1.3).
Figure 1.3 Formation of an NMR signal.
In this way a spectrum can be generated for a molecule containing atoms whose nuclei have non-zero magnetic moments. Among these nuclei are the proton, 1H, the fluorine nucleus, 19F, the nitrogen isotopes, 14N and 15N, and many others of chemical interest. However, the carbon nucleus, 12C, that is so important in organic chemistry has, like all other nuclei with even mass and even atomic number, no magnetic moment. Therefore, NMR studies with carbon are limited to the stable isotope 13C, which has a natural abundance of only 1.1%.
To illustrate a NMR spectrum and its essential characteristics, the proton NMR spectrum of ethyl formate is reproduced in Figure 1.4. The spectrum was measured in a magnetic field of 1.4 T with a frequency ν of 60 MHz. In addition to the resonance signals observed at different frequencies, it shows a step curve produced by an electronic integrator. The heights of the steps are proportional to the areas under the corresponding spectral lines.
Figure 1.4 1H NMR spectrum of ethyl formate.
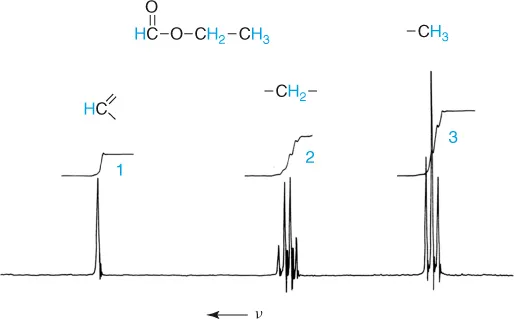
The following points should be noted:
1. Different resonance signals or groups of resonance signals are found for the protons. These arise because the protons reside in different chemical environments. The resonance signals are separated by a so-called chemical shift.
2. The area under a resonance signals is proportional to the number of protons that give rise to the signals. It can be measured by integration.
3. Not all proton resonances are simple (i.e., singlets). For some, characteristic splitting patterns are followed, forming triplets or quartets. This splitting is the result of spin–spin coupling – a magnetic interaction between different nuclei.
Empirically determined correlations between the spectral parameters, chemical shift and spin–spin coupling, on the one hand, and the structure of chemical compounds on the other hand form the basis for the application of proton and, in general, NMR to the structure determinations of unknown samples. In this respect the nuclear magnetic moment has proved itself to be a very sensitive probe with which one can gather extensive information. Thus, the chemical shift characterizes the chemical environment of the nucleus that is responsible for a signal. Integration of the spectrum allows one to draw conclusions concerning the relative numbers of nuclei present. Spin–spin coupling makes it possible to define the positional relationship between the nuclei since the magnitude of this interaction – the coupling constant J – depends upon the number and type of bonds separating them. The multiplicity of the resonance signals and the intensity distribution within the multiplet are, moreover, in simple cases, as illustrated by the ethyl group of ethyl formate, clearly dependent upon the number of nuclei on the neighboring group.
Numerous additional applications of NMR have been developed. One of general importance is based on the observation that the NMR spectra of many compounds are temperature dependent and apparently sensitive to dynamic processes. Such a case is found with dimethylformamide, the spectrum of which shows a doublet for the resonance of the methyl protons at 40oC while at 160oC a singlet is observed (Figure 1.5).
Figure 1.5 Temperature dependence of the 1H NMR spectrum of N,N-dimethylformamide.
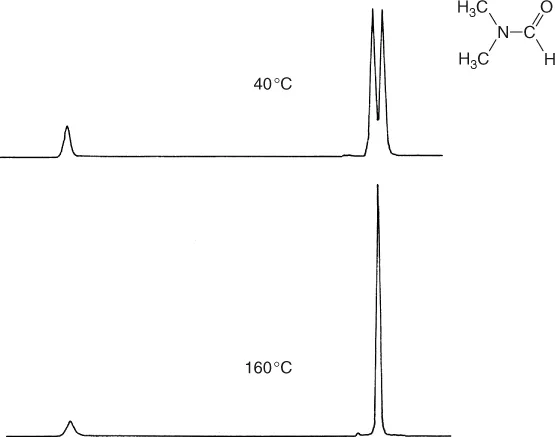
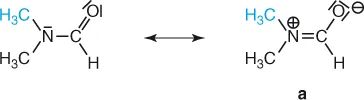
The cause of this different behavior at the two temperatures is the high barrier to rotation about the carbonyl carbon–nitrogen bond (88 kJ mol−1), which possesses partial double bond character as illustrated by the resonance form (a). The two methyl groups therefore have a relatively long life-time in different chemical environments, cis or trans to the carbonyl oxygen, and this leads to separate resonances. At higher temperatures the rate of internal rotation is increased and frequent interconversion of methyl groups between chemically different positions results, so that we are obviously no longer able to distinguish between them.
It follows that, for several molecules, the line shape of NMR signals is dependent upon dynamic processes and the rates of such processes can be studied with the aid of NMR spectroscopy. What is even more significant is that one can study fast reversible reactions that cannot be followed by means of classical kinetic methods. Thus, the progress achieved in the fields of fluxional molecules, like bullvalene, and in other areas, such as conformational analysis, would have been unimaginable without NMR spectroscopy.
NMR spectroscopy is also used successfully to study reaction mechanisms in all branches of chemistry. In these experiments, magnetic isotopes of hydrogen, carbon, or nitrogen (2H, 13C, 15N) and many others can be used in labeling experiments that are devised to follow the fate of a particular atom during the reaction of interest. Labeling with radioactive carbon, 14C, can be replaced today in many cases by labeling experiments with the stable but NMR active carbon isotope 13C. Only where the highest sensitivity is indispensable does the use of the radiocarbon method still p...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Preface
- Chapter 1: Introduction
- Part I Basic Principles and Applications
- Part II Advanced Methods and Applications
- Appendix
- Solutions for Exercises
- Glossary
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access NMR Spectroscopy by Harald Günther in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Spectroscopy & Spectrum Analysis. We have over one million books available in our catalogue for you to explore.