
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Practical HPLC Method Development
About this book
This revision brings the reader completely up to date on the evolving methods associated with increasingly more complex sample types analyzed using high-performance liquid chromatography, or HPLC. The book also incorporates updated discussions of many of the fundamental components of HPLC systems and practical issues associated with the use of this analytical method. This edition includes new or expanded treatments of sample preparation, computer assisted method development, as well as biochemical samples, and chiral separations.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
GETTING STARTED
1.1 Introduction
1.2 What is Known Before Starting
1.2.1 Nature of the Sample
1.2.2 Separation Goals
1.3 Sample Pretreatment and Detection
1.4 Developing the Separation
1.4.1 Selecting an HPLC Method and Initial Conditions
1.4.2 Getting Started on Method Development
1.4.3 Improving the Separation
1.4.4 Repeatable Separation
1.5 Completing the HPLC Method
1.5.1 Quantitation and Method Validation
1.5.2 Checking for Problems
1.5.3 Method Ruggedness
1.1 INTRODUCTION
Every day many chromatographers face the need to develop a high-performance liquid chromatography (HPLC) separation. Whereas individual approaches may exhibit considerable diversity, method development often follows the series of steps summarized in Fig. 1.1. In this chapter we review the importance of each of these steps, in preparation for a more detailed examination in following chapters.
FIGURE 1.1 Steps in HPLC method development.
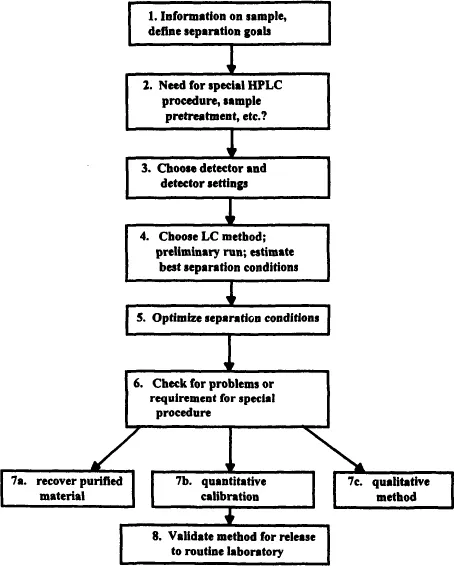
Our philosophy of method development is based on several considerations. There exists today a good practical understanding of chromatographic separation and how it varies with the sample and with experimental conditions. Any systematic approach to HPLC method development should be based on this knowledge of the chromatographic process. In most cases, a desired separation can be achieved easily with only a few experiments. In other cases, a considerable amount of experimentation may be needed. A good method-development strategy should require only as many experimental runs as are necessary to achieve the desired final result.
Ideally, every experiment will contribute to the end result so that there are no wasted runs. Usually, this requires that the results of each chromatographic run be assessed before proceeding with the next experiment. Sometimes the chemical structures of the sample components are known, other times this is not the case. The method-development scheme described in this book will usually work in either situation. Finally, method development should be as simple as possible, yet it should allow the use of sophisticated tools such as computer modeling (Chapter 10) if these are available.
1.2 WHAT IS KNOWN BEFORE STARTING
1.2.1 Nature of the Sample
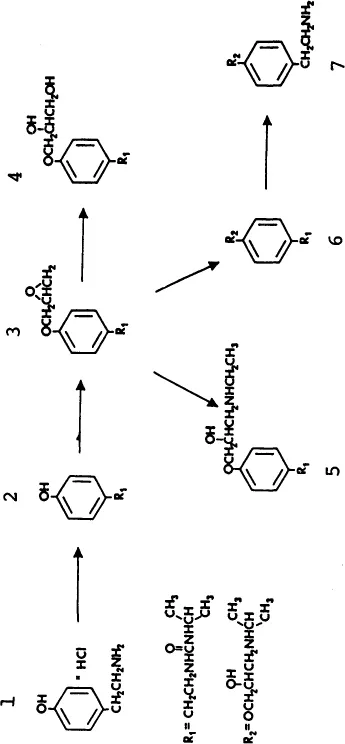
Before beginning method development, we need to review what is known about the sample. The goals of the separation should also be defined at this point. The kinds of sample-related information that can be important are summarized in Table 1.1. Ideally, a complete description of the sample is available; for example, an antihistamine tablet contains the active ingredient and various water-soluble excipients. The goal of HPLC separation in this case might be an assay of antihistamine content, so the primary interest is in the properties of the antihistamine that will affect its HPLC separation. Another situation might require analyzing a raw material for its major component and any contaminants. An example is provided by Fig. 1.2, which shows possible components of crude samples of the pharmaceutical product pafenolol (compound 6). In this case the chemical structures of possible contaminants can be inferred from the synthetic route used to prepare pafenolol, together with known side reactions leading to by-products. A total of six compounds can be expected in pafenolol (compound 3 can be ruled out because of its instability).
Table 1.1 Important Information Concerning Sample Composition and Properties
| Number of compounds present Chemical structures (functionality) of compounds Molecular weights of compounds pKa values of compounds UV spectra of compounds Concentration range of compounds in samples of interest Sample solubility |
FIGURE 1.2 Compounds present in crude samples of pafenolol.
(Reprinted with permission from Ref. 1.)
The chemical composition of the sample can provide valuable clues for the best choice of initial conditions for an HPLC separation. Depending on the use made of this sample information, two somewhat different approaches to HPLC method development are possible. Some chromatographers try to match the “chemistry” of the sample to a best choice of initial HPLC conditions. To do this, they rely heavily on their own past experience (i.e., separation of compounds of similar structure) and/or they supplement this information with data from the literature. Other workers proceed directly to an initial chromatographic separation, paying little attention to the nature of the sample. These two kinds of HPLC method development might be characterized as theoretical vs. empirical. Once an initial separation has been carried out, the choice of ensuing experiments can be made on the basis of similar considerations (theoretical vs. empirical).
Either a theoretical or an empirical approach to HPLC method development can be successful, and a “best” strategy is often some blend of these two procedures. In this book we emphasize empirical procedures in combination with techniques for minimizing the number of required experimental runs. However, theoretical considerations and the chemical composition of the sample are not ignored. It should also be kept in mind that the composition of many samples is not fully known at the beginning of HPLC method development (e.g., samples containing impurities, degradation products, metabolites, etc.). In these cases an empirical approach may be the only option.
1.2.2 Separation Goals
The goals of HPLC separation need to be specified clearly. Some related questions that should be asked at the beginning of method development include:
- Is the primary goal quantitative analysis, the detection of an (undesired) substance, the characterization of unknown sample components, or the isolation of purified material? The use of HPLC to isolate purified sample components for spectral identification or other purposes is discussed in Chapter 13.
- Is it necessary to resolve all sample components? For example, it may be necessary to separate all degradants or impurities from a product for reliable content assay, but it may not be necessary to separate these degradants or impurities from each other. When the complete separation of a sample by means of a single HPLC run proves difficult, the separation of a smaller subset of sample components is usually much easier.
- If quantitative analysis is requested, what levels of accuracy and precision are required? A precision of ±1 to 2% for major components of a sample is usually achievable, especially if sample pretreatment is not required. Means for improving assay precision are discussed in Chapter 14.
- For how many different sample matrices should the method be designed? A particular compound may be present in different sample types (e.g., a raw material, one or more formulations, an environmental sample, etc.). Will more than one HPLC procedure be necessary? Is a single (or similar) procedure for all samples desirable?
- How many samples will be analyzed at one time? When a large number of samples must be processed at the same time, run time becomes more important. Sometimes it is desirable to trade a decrease in sample resolution for a shorter run time [e.g., by shortening the column or increasing flow rate (Section 2.3.3.1)]. When the number of samples for analysis at one time is greater than 10, a run time of less than 20 min often will be important.
- What HPLC equipment and operator skills are present in the laboratory that will use the final method? Can the column be thermostated, and is an HPLC system for gradient elution available? Will the method be run on equipment of different design and manufacture [especially older models with increased extracolumn band broadening (Section 2.3.3.3)]? What HPLC experience and academic training do the operators have?
Agreement on what is required of the method should be obtained before method development begins.
1.3 SAMPLE PRETREATMENT AND DETECTION
Samples come in various form...
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Preface
- Glossary of Symbols and Terms
- Chapter 1: Getting Started
- Chapter 2: Basics of Separation
- Chapter 3: Detection Sensitivity and Selectivity
- Chapter 4: Sample Preparation
- Chapter 5: The Column
- Chapter 6: Non-Ionic Samples: Reversed-and Normal-Phase HPLC
- Chapter 7: Ionic Samples: Reversed-Phase, Ion-Pair, and Ion-Exchange HPLC
- Chapter 8: Gradient Elution
- Chapter 9: Systematic Approach to the Reversed-Phase Separation of Regular Samples
- Chapter 10: Computer-Assisted Method Development
- Chapter 11: Biochemical Samples: Proteins, nucleic Acids, Carbohydrates, and Related Compounds
- Chapter 12: Chiral Separations
- Chapter 13: Preparative HPLC Separation
- Chapter 14: Quantitation (Including Trace Analysis)
- Chapter 15: Completing the Method: Validation and Transfer
- Appendix I: Plate Number and Resolution
- Appendix II: Properties of Solvents Used in HPLC
- Appendix III: Retention in Reversed-Phase and Normal-Phase HPLC as a Function of Sample Molecular Structure
- Appendix IV: Preparing Buffered Mobile Phases
- Appendix V: Characterizing the Differences Among C8 or C18 Reversed-Phase Columns from Different Suppliers
- Appendix VI: Adjusting Mobile-Phase Water Content for Normal-Phase HPLC
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Practical HPLC Method Development by Lloyd R. Snyder,Joseph J. Kirkland,Joseph L. Glajch in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.