
- 352 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
In an effort to provide alternatives to trans and saturated fats, scientists have been busy modifying the physical properties of oils to resemble those of fats. In this fashion, many food products requiring a specific texture and rheology can be made with these novel oil-based materials without causing significant changes to final product quality. The major approach to form these materials is to incorporate specific molecules (polymers, amphiphiles, waxes) into the oil components that will alter the physical properties of the oil so that its fluidity will decrease and the rheological properties will be similar to those of fats. These new oilbased materials are referred to as oil gels, or "oleogels," and this emerging technology is the focus of many scientific investigations geared toward helping decrease the incidence of obesity and cardiovascular disease.
- Presents a novel strategy to eliminate trans fats from our diets and avoid excessive amounts of saturated fat by structuring oil to make it behave like crystalline fat
- Reviews recent advances in the structuring of edible oils to form new mesoscale and nanoscale structures, including nanofibers, mesophases, and functionalized crystals and crystalline particles
- Identifies evidence on how to develop trans fat free, low saturate functional shortenings for the food industry that could make a major impact on the health characteristics of the foods we consume
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Edible Oleogels by Alejandro G. Marangoni,Nissim Garti in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Food Science. We have over one million books available in our catalogue for you to explore.
Information
1
An Overview of the Past, Present, and Future of Organogels
Alejandro G. Marangoni1 and Nissim Garti2, 1Canada Research Chair in Food and Soft Materials Science, Guelph-Waterloo Physics Institute, Department of Food Science, University of Guelph, Guelph, Ontario, Canada; 2Ratner Chair of Chemistry, Casali Institute of Applied Chemistry, Institute of Chemistry, Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Jerusalem, Israel
Introduction
For over twenty years, clinical and epidemiological research has suggested that there is a positive relationship between trans fatty acid intake and a decrease in serum HDL (“good” cholesterol) in combination with an increase in serum LDL (“bad” cholesterol) (Mensink & Katan, 1990; Zock & Katan, 1992; Judd et al., 1994; Ascherio et al., 1999; Institute of Medicine, 2002; Mensink et al., 2003). Together these effects increase the risk of coronary heart disease. Both the Institute of Medicine and the American Heart Association recommend a reduction in the intake of trans fatty acids, preferably eliminating them from the diet (Institute of Medicine, 2002; American Heart Association, 2004). However, most consumers would find this difficult to achieve without appropriate labeling of staple foods. Prompted originally by the Center for Science in the Public Interest in 1994, the U.S. Food and Drug Administration (FDA) decreed that as of January 2006, food manufacturers must include the trans fatty acid content in product labels (US FDA 2003a; US FDA, 2003b).
This FDA ruling is an important milestone for public health, but it creates serious technological hurdles for the food manufacturing industry—it is difficult to eliminate trans fats from a food formulation. At the core of the problem is the ability to transform an oil (liquid at room temperature) to a fat (“solid” at room temperature). The difference between an oil and a fat is subtle. Oils and fats are mostly composed of molecules called triaclyglycerols: three fatty acids esterified onto a glycerol backbone. Whether such material is solid or liquid at a particular temperature depends on the chemical nature and physical properties of the constituent fatty acids.
Hydrogenation has been successfully commercially used for almost 100 years to transform oils into fats. From the time the British patent on liquid phase hydrogenation was issued to Norman in 1903 and its introduction in the U.S. in 1911, few chemical processes have made as great an economic impact on any industry. Hydrogenation opened new markets for vegetable-oil-based specialty products (O’Brien, 2003). Three reactions take place during hydrogenation: (1) the saturation of carbon-carbon double bonds, (2) the conversion of cis geometric isomers into more stable trans isomers, and (3) the creation of new positional isomers, where double bonds are shifted to new positions along the fatty acid chain. Both the saturation of double bonds as well as the cis to trans isomerization of double bonds will increase the melting point of a fat. Thus, cooling of this hydrogenated fat below the melting point of the newly created triacylglycerol species containing saturated and trans fatty acids will lead to the partial crystallization of the material. The resulting semisolid fat matrix will be a network of fat crystal aggregates with liquid oil trapped inside (Narine & Marangoni, 1990a). The solid-like characteristics of this material are due to this underlying fat crystal network (Narine & Marangoni, 1990b; Marangoni, 2000; Marangoni & Rogers, 2003). The presence or absence of this network of crystallized fat determines whether the material is a fat or an oil, respectively. Thus, the only previously known way to provide structure an oil, and thus convert it into a plastic fat, is by addition of high-melting temperature saturated or trans fats. This represents a major problem since fats containing high amounts of trans and saturated fatty acids are known to be atherogenic—they contribute to the build-up of cholesterol and other substances in artery walls. The American Heart Association discourages the consumption of excessive amounts of saturated animal and vegetable fats such as milk fat, palm oil, palm kernel oil, and coconut oil, as well as trans fats (American Heart Association, 2004). A new strategy for structuring edible oils is thus required.
In recent years, scientists have modified the physical properties of oils, which have a low viscosity and no elasticity, to resemble those of fats, which have a solid-like character and are elasto-plastic. Many food products that require a specific texture and rheology can be made with these novel oil-based materials without causing significant changes to final product quality. The major approach to forming these novel oil-based materials is to incorporate oil components that by various molecular interactions will alter the physical properties of the oil so that its fluidity will decrease and the rheological properties will be similar to those of fats. The continuous phase of these oil gels is lipidic, and they exhibit the characteristic physical properties of hydrogels. To distinguish these food oil gels from traditional “organogels”, which are usually gels of organic solvents used in various industrial applications in the chemical industry, we call these edible lipid oil gels, oleogels.
Several approaches have been taken to construct oleogels from vegetable oils and the research experience has brought us to a new area in which our food products will be healthier with no trans and minimal saturated fats. Before introducing the contributors to this book, we will briefly review the definition of a “fat” and some of the strategies that can be used to gel oils in the context of specific examples. Then, some of the applications of organogels will be summarized. Finally the chapters presented in this book will be introduced.
Fat Crystal Networks
For semisolid fat products (i.e., ice cream, margarine, and chocolate), the solid lipids, which normally exist as a three-dimensional (3D) colloidal fat crystal network, determine the physical properties of the product. Upon crystallization, hardstock triacylglycerols aggregate to form fat crystals similar to colloidal gels. The resulting clusters aggregate into flocs. The weak links between the flocs in the final macroscopic network are portrayed in Fig. 1.1.
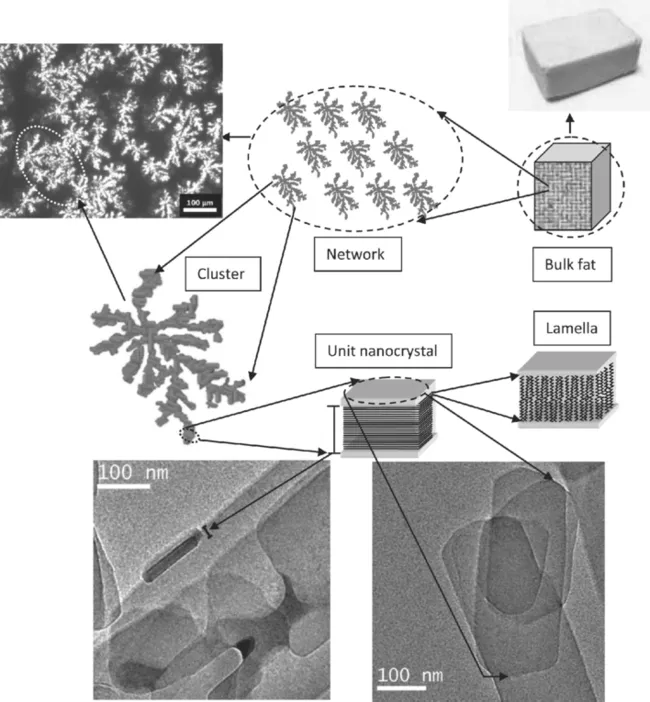
Heat, mass, and momentum transfer conditions have significant effects on the final microstructure and resultant macroscopic physical properties (i.e., hardness, yield stress, and compressibility) of fat products. For example, many studies have shown that the hardness of a fat crystal network is directly correlated to hardness determined by sensory analysis, and thus the sensory perception of the food product (Rousseau & Marangoni, 1998).
Soft, plastic materials, including fats, have different levels of structure present and each level influences the macroscopic properties of the material (Tang & Marangoni, 2006). The rheological properties of fats are the result of the combined effects of solid fat content (SFC), polymorphism, and fat crystal network microstructure, including the shape, size, area fraction, and the distribution pattern of the fat crystals (Tang & Marangoni, 2006). Since the hardstock triacylglycerols (TAGs) are responsible for the network structure, it is often difficult or impossible to eliminate these ingredients to improve ...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- Chapter 1: An Overview of the Past, Present, and Future of Organogels
- Chapter 2: Novel Strategies for Nanostructuring Liquid Oils into Functional Fats
- Chapter 3: Edible Oil Organogels Based on Self-assembled β-sitosterol + γ-oryzanol Tubules
- Chapter 4: Vegetable Oil-based Ricinelaidic Acid Organogels—Phase Behavior, Microstructure, and Rheology
- Chapter 5: Hydroxystearic Acid Oleogels
- Chapter 6: Candelilla Wax as an Organogelator for Vegetable Oils—An Alternative to Develop Trans-free Products for the Food Industry
- Chapter 7: Physical Properties of Organogels Made of Rice Bran Wax and Vegetable Oils
- Chapter 8: Monoglycerides in Oils
- Chapter 9: Physical Properties of β-fat Gel Made of Fully-hydrogenated Rapeseed Oil and Vegetable Oils
- Chapter 10: Ceramide Oleogels
- Chapter 11: Oleogels Based on Non-lamellar Lyotropic Liquid Crystalline Structures for Food Applications
- Chapter 12: Protein-templated Oil Gels and Powders
- Chapter 13: Ethylcellulose Oleogels
- Chapter 14: Clinical Study on 12-hydroxystearic Acid Organogel Ingestion
- Editors and Contributors
- Index