
Air Quality Management
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Air Quality Management
About this book
Designed to accompany the new Open University course in Environmental Monitoring and Protection, this is one of four new titles which will equip the reader with the tools to undertake Environmental Impact Assessments (EIAs). Used in planning, decision-making and management, EIAs review both the theoretical principles and environmental considerations of engineering and environmental projects to help steer fundamental legislation in the right direction. Air Quality Management begins with an introduction to the atmosphere around us and the units of concentration. It then discusses the importance of meteorology and the part it plays in air quality, before detailing the main types of air pollutants, their sources, and their effects on humans and their environments. Further chapters discuss measurement technologies and systems, as well as a selection of control and elimination methods. Finally, the book details methods of modelling atmospheric dispersion.
Discover our e-book series on Environmental Monitoring and Protection, published in partnership with The Open University!
Find out more about the series editors, the titles in the series and their focus on water, noise, air and waste, and The Open University courses in Environmental Management.
Visit www.wiley.com/go/ouebookseries
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Section 1: Air basics
1.1 Introduction


1.2 Clean air – a basic human need
| Activity | l min−1 | l hour−1 |
|---|---|---|
| Resting | 7.4 | 444 |
| Doing light work | 28 | 1680 |
| Doing heavy work | 43 | 2580 |
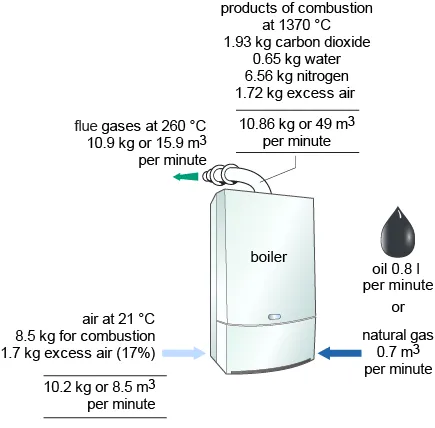
Determination of the stoichiometric (theoretical) air/fuel ratio for the complete combustion of petrol
SAQ 1
1.3 What is air pollution?

Table of contents
- Section 1: Air basics
- Section 2: Meteorology and air pollutants
- Section 3: Environmental monitoring
- Section 4: Air pollution control techniques
- Section 5: Atmospheric dispersion modelling
- Glossary
- References
- Acknowledgements
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app