
eBook - ePub
Handbook of Food Science and Technology 1
Food Alteration and Food Quality
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Food Science and Technology 1
Food Alteration and Food Quality
About this book
This book serves as a general introduction to food science and technology, based on the academic courses presented by the authors as well as their personal research experiences.
The authors' main focus is on the biological and physical-chemical stabilization of food, and the quality assessment control methods and normative aspects of the subsequent processes.
Presented across three parts, the authors offer a detailed account of the scientific basis and technological knowledge needed to understand agro-food transformation. From biological analyses and process engineering, through to the development of food products and biochemical and microbiological changes, the different parts cover all aspects of the control of food quality.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of Food Science and Technology 1 by Romain Jeantet, Thomas Croguennec, Pierre Schuck, Gérard Brulé, Romain Jeantet,Thomas Croguennec,Pierre Schuck,Gérard Brulé in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Food Science. We have over one million books available in our catalogue for you to explore.
Information
PART 1
Water and Other Food Constituents
1
Water
Water is the most abundant constituent of the majority of foods. It therefore plays a crucial role in the physicochemical characteristics and properties of the plant and animal foods we eat. These characteristics can be desired due to their contribution to food quality (the texture of fruit, vegetables and meat, which depends, among other things, on cell turgidity as well as on specific and complex interactions between water and other constituents). However, they can also contribute to food spoilage through biochemical and microbiological processes. As a result, several food preservation methods are based, at least partially, on lowering the water activity (aw) or the water availability.
1.1. Structure and state of water
The water molecule, composed of two hydrogen atoms and one oxygen atom (H2O), can exist, like many substances, in three different states: solid, liquid or gas.
In the liquid and vapor state, the water molecule is a polar monomer (see Figure 1.1).
In the solid state (i.e. ice), water molecules are linked by hydrogen bonds and form a crystalline polymer in which each monomer molecule is connected to four other molecules by hydrogen bonds. The distance between two oxygen atoms is 0.276 nm. At temperatures below −173°C, all hydrogen atoms are involved in hydrogen bonds, whereas at 0°C only around 50% are involved, and at 100°C only a small percentage are involved.
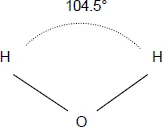
Figure 1.1. Water molecule
Certain water properties can be attributed to these intermolecular bonds, in particular the boiling point, melting point, latent heat of fusion, latent heat of vaporization, specific heat, surface tension and the dielectric constant. However, water in a liquid state behaves like a monomer in terms of viscosity and the diffusion coefficient (Tables 1.1 and 1.2).
Table 1.1 Properties of water
| Properties | Unit | Value |
| Molar mass | g mol–1 | 18.01528 |
| Melting point (at 101,325 Pa) | °C | 0.00 |
| Boiling point (at 101,325 Pa) | °C | 100.00 |
| Maximum density | kg m–3 | 999.95 |
| Temperature of maximum density | °C | 4.00 |
| Triple-point temperature | °C | 0.01 |
| Triple-point density (liquid) | kg m–3 | 999.78 |
| Triple-point density (gas) | 10−3 kg m–3 | 4.88 |
| Latent heat of sublimation at the triple point | 103 J kg–1 | 2800 |
| Critical temperature | °C | 373.99 |
| Critical pressure | MPa | 22.064 |
| Critical density | kg m–3 | 322 |
| Specific volume at the critical point | 10−3 m3 kg–1 | 3.11 |
| Latent heat of freezing at 0°C | 103 J kg–1 | 335 |
Different theoretical models have been proposed to explain the liquid and solid state behavior of water. Monomers as well as higher-energy molecules exist in a static equilibrium: each molecule is involved in one to four hydrogen bonds; the latter can form short-lived labile clusters.
Table 1.2. Properties of water
| Properties | Units | Frozen water | Liquid water | Water vapor | |||||||
| Temperature | (°C) | –20 | 0 | 0 | 20 | 40 | 60 | 80 | 100 | 100 | 200 |
| Density | kg m–3 | 919.3 | 916.8 | 999.8 | 998.2 | 992.2 | 983.2 | 971.8 | 958.4 | 0.589 | 0.452 |
| Viscosity | 10–6 Pa s | – | – | 1,793 | 1,002 | 653 | 466 | 354 | 281 | 12.5 | 16.4 |
| Surface tension | 10–3 N m–1 | – | – | 75.64 | 72.75 | 69.60 | 66.24 | 62.67 | 58.91 | – | – |
| Vapor pressure | (Pa) | 103.4 | 611.3 | 611.3 | 2,338.8 | 7,381.4 | 19,932 | 47... | |||
Table of contents
- Cover
- Table of Contents
- Title
- Copyright
- Introduction
- PART 1: Water and Other Food Constituents
- PART 2: Food Modifying Agents and Mechanisms
- PART 3: Quality Control and Assessment
- Bibliography
- List of Authors
- Index
- End User License Agreement