
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Spectroscopy
About this book
This second, thoroughly revised, updated and enlarged edition provides a straightforward introduction to spectroscopy, showing what it can do and how it does it, together with a clear, integrated and objective account of the wealth of information that may be derived from spectra. It also features new chapters on spectroscopy in nano-dimensions, nano-optics, and polymer analysis.
Clearly structured into sixteen sections, it covers everything from spectroscopy in nanodimensions to medicinal applications, spanning a wide range of the electromagnetic spectrum and the physical processes involved, from nuclear phenomena to molecular rotation processes.
In addition, data tables provide a comparison of different methods in a standardized form, allowing readers to save valuable time in the decision process by avoiding wrong turns, and also help in selecting the instrumentation and performing the experiments.
These four volumes are a must-have companion for daily use in every lab.
Clearly structured into sixteen sections, it covers everything from spectroscopy in nanodimensions to medicinal applications, spanning a wide range of the electromagnetic spectrum and the physical processes involved, from nuclear phenomena to molecular rotation processes.
In addition, data tables provide a comparison of different methods in a standardized form, allowing readers to save valuable time in the decision process by avoiding wrong turns, and also help in selecting the instrumentation and performing the experiments.
These four volumes are a must-have companion for daily use in every lab.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Preparation of Liquid and Solid Samples
1.1 Introduction
Sampling and sample preparation of liquids and solids often present significant challenges for real-world quantitative analyses using spectrometric techniques (e.g., UV–vis and infrared absorption, luminescence and Raman spectroscopies). Very often, the native form of a sample is unsuitable for analysis. This could be due to (i) the complex nature of the object, which could provide false measurements due to interferences or masking agents; (ii) the size of the object being too large to analyze in its entirety (e.g., laboratory sample of contaminated soil); or (iii) the awkward shape of the object, preventing it from fitting within the instrument in which the measurement is to be made. To overcome these problems, some sort of sample preparation must be performed. In many cases, sample preparation is required before any quantitative analysis, and both can have dramatic impacts on the measured results and their accuracy.
The previous chapter presented the main criteria to be followed for solid and liquid sample collection. This chapter presents a general overview of various methods for sample preparation. This general topic has been described extensively in a variety of research papers and review chapters in the literature 1–6, with specific variations for particular applications often being necessary. This chapter deals with the sample preparation required to provide a material suitable for spectrometric analysis.
1.2 Preparation of Samples for Analysis
1.2.1 Measurement Process
Samples collected for spectral analysis can generally be classified into three categories based on their state: (i) solids, (ii) liquids, and (iii) gases. Samples are typically classified on the basis of their state as a method of providing an initial means of handling/treating them. A general flow diagram depicting the measurement process for various samples is shown in Figure 1.1.
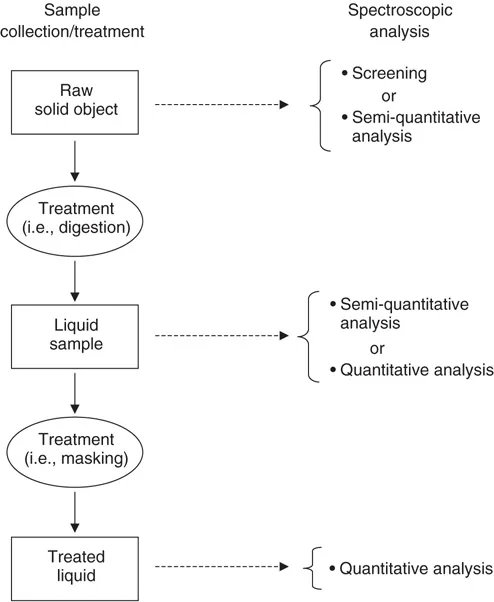
Figure 1.1 Schematic diagram depicting generalized sample preparation and analysis steps.
1.2.2 Preparation of Samples for Analysis
Once a representative sample has been obtained from the object of interest, the next step is to prepare the sample for analysis. Since sample preparation depends upon both the analyte (e.g., iron in water, polycyclic aromatic compounds in benzene, etc.) and the instrumentation used to perform the spectroscopic measurement (e.g., UV–vis or IR absorption, luminescence, Raman, HPLC-fluorescence, GC–MS, etc.), details of the preparation process will vary from analysis to analysis. Many general procedures have been developed over the years for the preparation of various types of samples prior to analysis. Most of these procedures can be classified depending upon the type of samples that are to be analyzed, either solid or liquid. Within each of these categories exist several subcategories based upon the type of analyte to be measured.
1.2.3 Solid Samples
The first of the two categories that we will discuss is solid samples. The various types of solid samples that are most often encountered have been discussed elsewhere in the sampling section of this chapter (i.e., powders, chunks, or cores). In the case of the latter two sample types (chunks and cores), the first preparation step involves grinding the larger pieces into a powder, which is much easier to deal with, and introduce it into the measuring instrument. The most common method for obtaining powders from these samples involves grinding a solid sample into a powder using either a mortar and pestle or a ball mill. Mortars typically come in two different types: the agate version (or ceramic) for relatively soft solid materials (e.g., large crystalline substances) that must be ground into a fine powder; or steel mortars, which are used for crushing much harder materials. In agate mortars, the material to be ground is placed in the depression of the mortar and then the sample is pressed down with the pestle in a rotating movement. When using agate or ceramic mortars and pestles, it is important to clean them thoroughly to avoid sample contamination. Less expensive mortars are typically softer and hence can be scratched more easily than more expensive ones. This is especially the case for ceramic mortars. Once scratched, they are much more difficult to clean, and may require the use of an abrasive or even a strong HCl solution. Steel mortars, also known as percussion mortars, have a hardened steel sleeve and pestle that fit snuggly into the mortar, and a hammer is then used to strike the pestle and subsequently crush the sample.
Another grinding tool that is often used to grind solid samples is the ball mill. A ball mill is a ceramic drum within which are placed the sample and many small balls made of hard ceramic. To grind the sample, the drum is rotated, producing a very fine powder. Ball mills are often used on softer solids, as the time taken for grinding is directly proportional to the hardness of the material. To ensure that none of the material that is being ground sticks to the walls of the mill during the grinding process, thereby producing larger pieces, the samples are typically dried to 100–110 °C prior to grinding to expel any water.
1.2.3.1 Sample Preparation for Inorganic Analysis
Most commercially available instruments for quantitative analyses in chemical and biological sciences are designed for the analysis of liquid samples. Because of this, solid samples that are to be analyzed are typically dissolved in a suitable solvent, usually following conversion to a powder by one of the previously described methods. The solvent chosen for this dissolution process may be either polar (e.g., water) or nonpolar (e.g., benzene) depending on the polarity and reactivity of the sample. In order to ensure that the entire analyte has been dissolved in the solution of interest, a solvent is chosen that can dissolve the entire solid sample (analyte as well as other materials). If the sample cannot be readily dissolved in these mild conditions, many other techniques are available for dissolution. As inorganic materials often present the greatest difficulty in dissolution, this section will deal primarily with these materials.
1.2.3.1.1 Acid Digestion
Acid digestion of inorganic materials is a common alternative to the mild solvents used for dissolution, as described above. When using acids to digest metallic materials, great care should be taken not to change the speciation of the metal or metallic species to be analyzed. When analyzing a reduced state of a metal or metallic species, several nonoxidizing acids can be used. These include HF, HCl, HBr, H3PO4, dilute H2SO4, and dilute HClO4. These acids dissolve most metals with negative reduction potentials. However, in some cases (i.e., aluminum), a protective oxide layer is for...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- : Preface
- List of Contributors
- Chapter 1: Preparation of Liquid and Solid Samples
- Chapter 2: Liquid and Solid Sample Collection
- Chapter 3: Basics of Optical Spectroscopy
- Chapter 4: Instrumentation
- Chapter 5: Measurement Techniques
- Chapter 6: Applications
- Chapter 7: An Introduction to Solution, Solid-State, and Imaging NMR Spectroscopy
- Chapter 8: Solution NMR Spectroscopy
- Chapter 9: Suspended-State NMR Spectroscopy (High-Resolution Magic Angle Spinning (HR-MAS) NMR Spectroscopy)
- Chapter 10: Solid-State NMR
- Chapter 11: Mass Spectrometry
- Chapter 12: Multiparametric Analysis of Mass Spectrometry-Based Proteome Profiling in Gestation-Related Diseases
- Chapter 13: Laser-Assisted Mass Spectrometry
- Chapter 14: X-ray Fluorescence Analysis
- Chapter 15: Atomic Absorption Spectrometry (AAS) and Atomic Emission Spectrometry (AES)
- Chapter 16: Inductively Coupled Plasma Spectrometry
- Chapter 17: Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICPMS)
- Chapter 18: Electron Probe Techniques
- Chapter 19: Ion/Neutral Probe Techniques
- Chapter 20: Photon Probe Techniques
- Chapter 21: Single-Molecule Spectroscopy
- Chapter 22: Single-Molecule Interfacial Electron-Transfer Dynamics
- Chapter 23: Scanning Near-Field Gap-Mode Microscopy
- Chapter 24: Trends in Bioanalytical Spectroscopy
- Chapter 25: Quality Assessment of Spectroscopic Methods in Clinical Laboratories
- Chapter 26: UV–Vis and NIR Fluorescence Spectroscopy
- Chapter 27: Principles of Vibrational Spectroscopic Methods and their Application to Bioanalysis
- Chapter 28: Bioanalytical NMR Spectroscopy
- Chapter 29: Direct Optical Detection in Bioanalytics
- Chapter 30: Surface Plasmon Spectroscopy Methods and Electrochemical Analysis
- Chapter 31: Applications of Fourier Transform Infrared (FTIR) Imaging
- Chapter 32: Photon Correlation Spectroscopy Coupled with Field-Flow Fractionation for Polymer Analysis
- Chapter 33: Surface Plasmon Resonance Spectroscopy and Molecularly Imprinted Polymer (MIP) Sensors
- Chapter 34: LC-MS in Environmental Analysis
- Chapter 35: Ion Attachment Mass Spectrometry for Environmental Analysis
- Chapter 36: Immunoassays
- Chapter 37: Process Control in Chemical Manufacturing
- Chapter 38: Process Control Using Spectroscopic Tools in Pharmaceutical Industry and Biotechnology
- Chapter 39: Applications of Optical Spectroscopy to Process Environments
- Chapter 40: Spectral Imaging in Quality and Process Control
- Chapter 41: Trends in Spectroscopic Techniques for Process Control
- Chapter 42: Optical Spectroscopy at Surfaces
- Chapter 43: NEXAFS Studies at Surfaces
- Chapter 44: The X-Ray Standing Wave Technique
- Chapter 45: Photoelectron Spectroscopy Applications to Materials Science
- Chapter 46: Miniaturized Optical Sensors for Medical Diagnostics
- Chapter 47: Tip-Enhanced Near-Field Optical Microscopy
- Chapter 48: Optical Waveguide Spectroscopy
- Chapter 49: Mass Spectral Detection
- Chapter 50: Optical Detection
- Chapter 51: Atomic Spectral Detection
- Chapter 52: NMR as a Chromatography Detector
- Chapter 53: Optical Spectroscopy
- Chapter 54: Nuclear Magnetic Resonance Spectroscopy
- Chapter 55: Mass Spectrometry
- Chapter 56: Raman Spectroscopy Fundamentals
- Index
- End User License Agreement
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Handbook of Spectroscopy by G¿nter Gauglitz,David S. Moore,Günter Gauglitz in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.