
- 432 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
In recent years, there have been considerable developments in techniques for the investigation and utilisation of enzymes. With the assistance of a co-author, this popular student textbook has been updated to include techniques such as membrane chromatography, aqueous phase partitioning, engineering recombinant proteins for purification and due to the rapid advances in bioinformatics/proteomics, a discussion of the analysis of complex protein mixtures by 2D-electrophoresis and RPHPLC prior to sequencing by mass spectroscopy. Written with the student firmly in mind, no previous knowledge of biochemistry, and little of chemistry, is assumed. It is intended to provide an introduction to enzymology, and a balanced account of all the various theoretical and applied aspects of the subject which are likely to be included in a course.
- Provides an introduction to enzymology and a balanced account of the theoretical and applied aspects of the subject
- Discusses techniques such as membrane chromatography, aqueous phase partitioning and engineering recombinant proteins for purification
- Includes a discussion of the analysis of complex protein mixtures by 2D-electrophoresis and RPHPLC prior to sequencing by mass spectroscopy
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part 1
Structure and Function of Enzymes
1
An Introduction to Enzymes
1.1 WHAT ARE ENZYMES?
Enzymes are biological catalysts. They increase the rate of chemical reactions taking place within living cells without themselves suffering any overall change. The reactants of enzyme-catalysed reactions are termed substrates. Each enzyme is quite specific in character, acting on a particular substrate or substrates to produce a particular product or products.
All enzymes are proteins. However, without the presence of a non-protein component called a cofactor, many enzyme proteins lack catalytic activity. When this is the case, the inactive protein component of an enzyme is termed the apoenzyme, and the active enzyme, including cofactor, the holoenzyme. The cofactor may be an organic molecule, when it is known as a coenzyme, or it may be a metal ion. Some enzymes bind cofactors more tightly than others. When a cofactor is bound so tightly that it is difficult to remove without damaging the enzyme, it is sometimes called a prosthetic group.
To summarize diagrammatically:
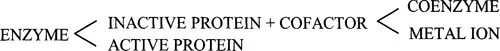
As we shall see later, both the protein and cofactor components may be directly involved in the catalytic processes taking place.
1.2 A BRIEF HISTORY OF ENZYMES
Until the nineteenth century, it was considered that processes such as the souring of milk and the fermentation of sugar to alcohol could only take place through the action of a living organism. In 1833, the active agent breaking down the sugar was partially isolated and given the name diastase (now known as amylase).
A little later, a substance which digested dietary protein was extracted from gastric juice and called pepsin. These and other active preparations were given the general name ferments. Justus von Liebig recognized that these ferments could be non-living materials obtained from living cells, but Louis Pasteur and others still maintained that ferments must contain living material.
While this dispute continued, the term ferment was gradually replaced by the name enzyme. This was first proposed by Wilhelm Kühne in 1878, and comes from the Greek, enzumé (έvξvμη), meaning ‘in yeast’. Appropriately, it was in yeast that a factor was discovered which settled the argument in favour of the inanimate theory of catalysis: brothers Eduard and Hans Büchner showed, in 1897, that sugar fermentation could take place when a yeast cell extract was added even though no living cells were present.
In 1926, James Sumner crystallized urease from jack-bean extracts and, in the next few years, many other enzymes were purified and crystallized. Once pure enzymes were available, their structure and properties could be determined, and the findings form the material for most of this book.
Today, enzymes still form a major subject for academic research. They are investigated in hospitals as an aid to diagnosis and, because of their specificity of action, are of great value as analytical reagents. Enzymes are still widely used in industry, continuing and extending many processes which have been used since the dawn of history.
1.3 THE NAMING AND CLASSIFICATION OF ENZYMES
1.3.1 Why classify enzymes?
There is a long tradition of giving enzymes names ending in ‘-ase’. The only major exceptions to this are the proteolytic enzymes, i.e. ones involved in the breakdown of proteins, whose names usually end with ‘-in’, e.g. trypsin.
The names of enzymes usually indicate the substrate involved. Thus, lactase catalyses the hydrolysis of the disaccharide lactose to its component monosaccharides, glucose and galactose:
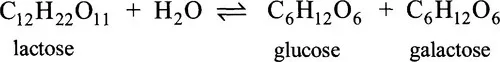
The name lactase is a contraction of the clumsy, but more precise, lactosase. The former is used because it sounds better but it introduces a possible trap for the unwary because it could easily suggest an enzyme acting on the substrate lactate. There is nothing in the name of this enzyme or many others to indicate the type of reaction being catalysed. Fumarase, for example, by analogy with lactase might be supposed to catalyse a hydrolytic reaction, but, in fact, it hydrates fumarate to form malate:
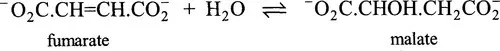
The names of other enzymes, e.g. transcarboxylase, indicate the nature of the reaction without specifying the substrates (which in the case of transcarboxylase are methylmalonyl-CoA and pyruvate). Some names, such as catalase, indicate neither the substrate nor the reaction (catalase mediates the decomposition of hydrogen peroxide).
Needless to say, whenever a new enzyme has been characterized, great care has usually been taken not to give it exactly the same name as an enzyme catalysing a different reaction. Also, the names of many enzymes make clear the substrate and the nature of the reaction being catalysed. For example, there is little ambiguity about the reaction catalysed by malate dehydrogenase. This enzyme mediates the removal of hydrogen from malate to produce oxaloacetate:
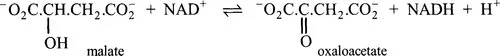
However, malate dehydrogenase, like many other enzymes, has been known by more than one name.
So, because of the lack of consistency in the nomenclature, it became apparent as the list of known enzymes rapidly grew that there was a need for a systematic way of naming and classifying enzymes. A commission was appointed by the International Union of Biochemistry (later re-named the International Union of Biochemistry and Molecular Biology, IUBMB), and its report, published in 1964, forms the basis of the currently accepted system. Revised editions of the report were published in 1972, 1978, 1984 and 1992. An electronic version is now maintained by the IUBMB on an accessible web-site, and this is updated on a regular basis.
1.3.2 The Enzyme Commission’s system of classification
The Enzyme Commission divided enzymes into six main classes, on the basis of the total reaction catalysed. Each enzyme was assigned a code number, consisting of four elements, separated by dots. The first digit shows to which of the main classes the enzyme belongs, as follows:
| First digit | Enzyme class | Type of reaction catalysed |
| 1 | Oxidoreductases | Oxidation/Reduction reactions |
| 2 | Transferases | Transfer of an atom or group between two molecules (excluding reactions in other classes) |
| 3 | Hydrolases | Hydrolysis reactions |
| 4 | Lyases | Removal of a group from substrate (not by hydrolysis) |
| 5 | Isomerases | Isomerization reactions |
| 6 | Ligases | The synthetic joining of two molecules, coupled with the breakdown of the pyrophosphate bond in a nucleoside triphosphate |
The second and third digits in the code further describe the kind of reaction being catalysed. There is no general rule, because the meanings of these digits are defined separately for each of the main classes. Some examples are given later in this chapter. Note that, for convenience, and in line with normal practice, some ...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Authors’ Preface
- Part 1: Structure and Function of Enzymes
- Part 2: Kinetic and Chemical Mechanisms of Enzyme-Catalysed Reactions
- Part 3: Application of Enzymology
- Answers to Problems
- Abbreviations
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Enzymes by T Palmer,P L Bonner in PDF and/or ePUB format, as well as other popular books in Tecnología e ingeniería & Ciencia de los alimentos. We have over one million books available in our catalogue for you to explore.