The Maudsley Prescribing Guidelines in Psychiatry
David M. Taylor, Carol Paton, Shitij Kapur, David M. Taylor, Carol Paton, Shitij Kapur
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
The Maudsley Prescribing Guidelines in Psychiatry
David M. Taylor, Carol Paton, Shitij Kapur, David M. Taylor, Carol Paton, Shitij Kapur
Über dieses Buch
Helps with complex prescribing needs
The evidence base for drug treatments in psychiatry ranges from meta-analyses andrandomised controlled clinical trials to single case reports, and from NICE guidelines to individual SPCs. Where do you look for information when transferring a patient from one drug to another? Where do you find a clear overview when dealing with a complex patient (e.g, with co-morbid epilepsy or liver disease or HIV infection)? Where can you seek advice on prescribing psychotropics during pregnancy? The Maudsley Prescribing Guidelines in Psychiatry! The leading clinical reference for handling prescribing problems as encountered in daily practice and for formulating prescribing policy.
Evidence-based and written by experts
This book is the essential guide for anyone responsible for prescribing, dispensing or administering drugs for patients with mental health disorders. All the evidence has been reviewed and summarized succinctly by an expert team of psychiatrists and pharmacists.
New content and improved format
This new edition makes greater use of tables and boxes to facilitate quick reference and includes new sections on cytochrome-mediated interactions and psychiatric side effects of non-psychotropic drugs.
Clinically relevant
Chapters address plasma monitoring, schizophrenia, bipolar disorder, depression and anxiety, children and adolescents, substance abuse and special patient groups. Each section has a full reference list. The book covers prescribing drugs outside their licensed indications and their interaction with substances such as alcohol, nicotine and caffeine.
Useful for all levels of experience
Trainees will gain important information regarding the rational, safe and effective use of medications for patients with mental illness. Experienced clinicians will find excellent guidance regarding more complex issues that they may not encounter regularly.
Why the Maudsley Prescribing Guidelines in Psychiatry?
Long recognized as an international trailblazer in mental health care, the Maudsley Hospital earned its reputation for excellence in both in-patient and community care. It is highly regarded for its research, and pioneered the use of clinical neuroscience. You can trust The Maudsley Prescribing Guidelines in Psychiatry to be scientifically sound and clinically effective.
Häufig gestellte Fragen
Information
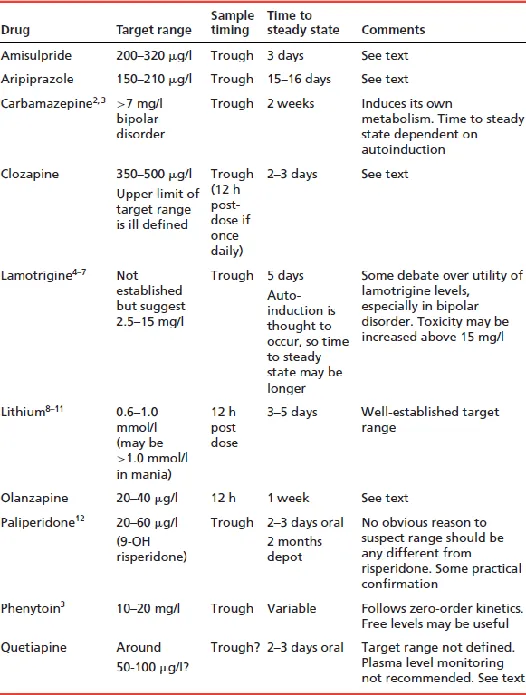
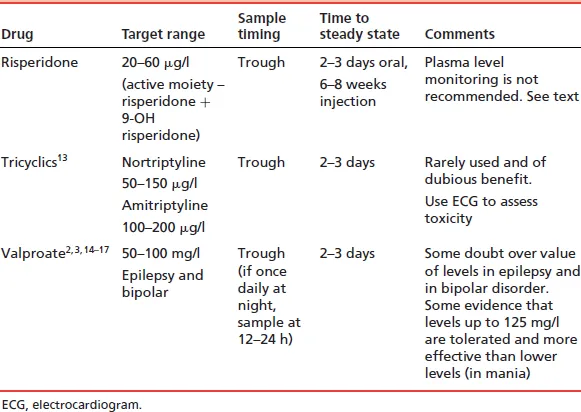
- Is there a clinically useful assay method available? Only a minority of drugs have available assays. The assay must be clinically validated and results available within a clinically useful timescale.
- Is the drug at ‘steady state’? Plasma levels are usually meaningful only when samples are taken after steady-state levels have been achieved. This takes 4–5 drug half-lives.
- Is the timing of the sample correct? Sampling time is vitally important for many but not all drugs. If the recommended sampling time is, say, 12 h post dose, then the sample should be taken 11–13 h post dose if possible; 10–14 h post dose, if absolutely necessary. For trough or ‘predose’ samples, take the blood sample immediately before the next dose is due. Do not, under any circumstances, withhold the next dose for more than 1 or (possibly) 2 h until a sample is taken. Withholding for longer than this will inevitably give a misleading result (it will give a lower result than ever seen in the usual, regular dosing), which may lead to an inappropriate dose increase. Sampling time is less critical with drugs with a long half-life (e.g. olanzapine) but, as an absolute minimum, prescribers should always record the time of sampling and time of last dose.
If a sample is not taken within 1–2 h of the required time, it has the potential to mislead rather than inform. The only exception to this is if toxicity is suspected – sampling at the time of suspected toxicity is obviously appropriate. - Will the level have any inherent meaning? Is there a target range of plasma levels? If so, then plasma levels (from samples taken at the right time) will usefully guide dosing. If there is not an accepted target range, plasma levels can only indicate adherence or potential toxicity. If the sample is being used to check compliance, bear in mind that a plasma level of zero indicates only that the drug has not been taken in the past several days. Plasma levels above zero may indicate erratic compliance, full compliance or even long-standing non-compliance disguised by recent taking of prescribed doses. Note also that target ranges have their limitations: patients may respond to lower levels than the quoted range and tolerate levels above the range; also, ranges quoted by different laboratories sometimes vary widely without explanation.
- Is there a clear reason for plasma level determination? Only the following reasons are valid:
- to confirm compliance (but see above)
- if toxicity is suspected
- if a pharmacokinetic drug interaction is suspected
- if clinical response is difficult to assess directly (and where a target range of plasma levels has been established)
- if the drug has a narrow therapeutic index and toxicity concerns are considerable.