
eBook - ePub
Theory of Flame Retardation of Polymeric Materials
Li Jianjun, Ou Yuxiang
This is a test
Buch teilen
- 288 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Theory of Flame Retardation of Polymeric Materials
Li Jianjun, Ou Yuxiang
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Flame retardant materials are of vital importance in guaranteeing personal security. Especially the demand for non-toxic, low smoking, polymerized flame retardants increases and new materials enter the market. The authors present the fundamental theory of polymer combustion, compare different flame retardants, describe smoke suppression mechanisms, and explain analyzing techniques for new materials.
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Theory of Flame Retardation of Polymeric Materials als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Theory of Flame Retardation of Polymeric Materials von Li Jianjun, Ou Yuxiang im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Technology & Engineering & Materials Science. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Generally speaking, the combustion process of polymers can be divided into five stages (Figure 1.1) [1] in time, which includes thermal decomposition, ignition, propagation, stable combustion and combustion attenuation. At the same time, these five stages can also be separated in space, such as surface-heating area, condensed phase transformation area (decomposition, crosslinking, carbonization) and vapor-phase combustible combustion area. Figure 1.2 shows the unit model of polymer combustion [2].
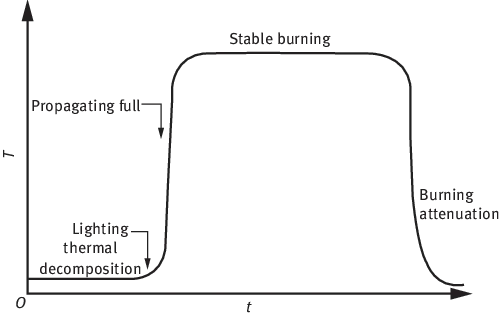
Figure 1.1: Five stages of polymer combustion [1].
Due to their characteristics, there will be some special thermal behaviors of polymer during the heating procedure, such as glass transitions, softening, melting, expansion, foaming, shrinkage, etc [3].
In this chapter, we will not only explain the five stages of polymer combustion but also analyze the smoke, toxic and corrosive products produced during the combustion procedure to provide some theoretical foundations for the discussion of the flame-retardation mechanisms of polymers.
1.1 Thermal decomposition
1.1.1 Overview
When the thermoplastics were heated, evaporation and pyrolysis of the solid phase were considered to be confined to a thin layer, namely condensed-phase/vapor-phase interface. Generally, when the polymer is heated, it will be first thermally degraded into pieces or monomers or fragments with low-molecular weight, and the escape rate of the latter is concerned with the mass transfer process [4, 5].
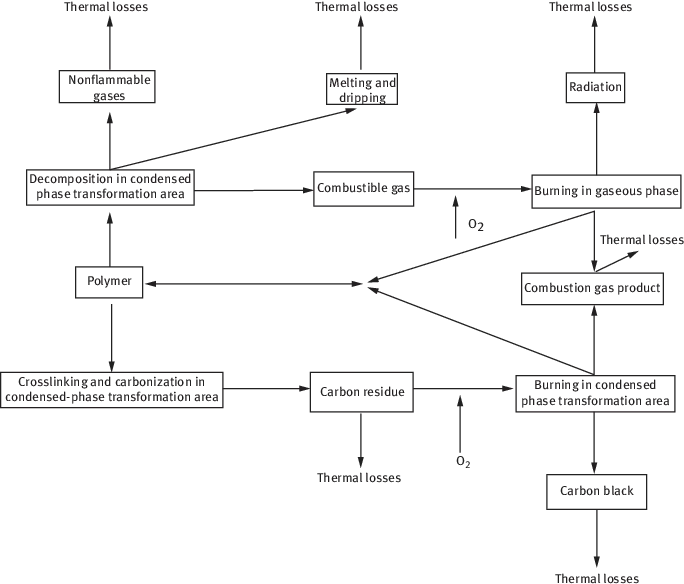
Figure 1.2: Unit model of polymer combustion [2].
The thermal decomposition of polymers is the first step for the cause and development of combustion, and it is very important for estimating the reaction of materials to fire, synthetizing heat-resistant polymer and recycling waste polymer.
When an external heat source is put on the material, the material temperature gradually rises. An external heat source may be produced directly from the flame (by radiation and convective heat transfer), the hot vapor (by conduction and convection heat transfer) and the hot solid material (by heat conduction). When heated, heating rate of material not only depends on external heat flow rate and temperature difference but also is related to specific heat capacity, thermal conductivity and carbonization, evaporation and other changing latent heat of the material.
Under the intense radiation near the hot objects, the surface of polymer will be heated rapidly, and its temperature rises with the square root of thermal radiation time. Taking polyethylene (PE) as an example, when the radiation intensity is 36 kW/M2 (which is equal to the blackbody temperature of 613 °C), its surface temperature rising based on time is shown in Figure 1.3 [6].
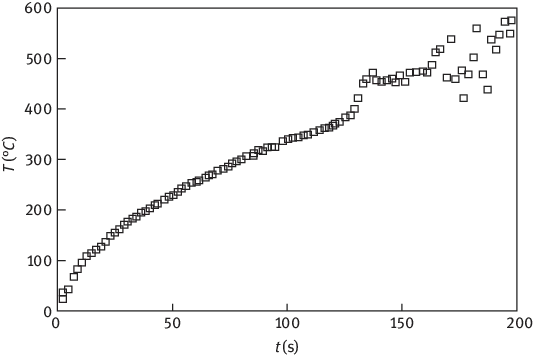
Figure 1.3: Relationship between polyethylene surface temperature and time [6] under a radiation intensity of 36 kW/M2.
If the radiation intensity is low, the highest surface temperature of polymer would not be enough to ignite polymer, as well as would have low thermal diffusivity and thin thermal layer. Thus, polymer is difficult to be ignited (the ignition temperature of polymethyl methacrylate [PMMA] is 250–350 °C, whereas that of PE is 330–370 °C), and only thermal decomposition will occur [7].
When the temperature of polymer rises up to a certain level, it begins to degrade, and the initial temperature of degradation is usually the temperature of bond rupture that has the worst thermal stability. During this period, polymer could still be intact, but the weakest bond ruptures often change the color and luster of polymers. There are two forms of degradation: nonoxide degradation and oxidative degradation. The former occurs without oxygen, whereas the latter should be heated and degraded with oxygen. Proportion of the most volatile decomposition temperature of the bonds and the instability of bonds in polymer are closely related to the degradation of polymer.
When most bonds rupture due to polymer decomposition, it enables a long chain of 104–105 carbon atoms to be decomposed into low molecular product. Then, the molecular weight of polymer gets smaller dramatically, polymer itself also begins to change, and this change can lead to a complete loss on its physical integrity or generate new substance with different properties, most of which are compounds with low molecular weight and many of these compound are inflammable materials, which can also volatilize into the vapor phase, thus reducing the total weight of polymer. For example, when thermal decomposition occurs, polyformaldehyde (POM) or PMMA can be decomposed into monomer formaldehyde or methyl methacrylate, respecti...