
eBook - ePub
Chiroptical Spectroscopy
Fundamentals and Applications
Prasad L. Polavarapu
This is a test
Condividi libro
- 430 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Chiroptical Spectroscopy
Fundamentals and Applications
Prasad L. Polavarapu
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Informazioni sul libro
This book details chiroptical spectroscopic methods: electronic circular dichroism (ECD), optical rotatory dispersion (ORD), vibrational circular dichroism (VCD), and vibrational Raman optical activity (VROA). For each technique, the text presents experimental methods for measurements and theoretical methods for analyzing the experimental data. It also includes a set of experiments that can be adopted for undergraduate teaching laboratories. Each chapter is written in an easy-to-follow format for novice readers, with necessary theoretical formalism in appendices for advanced readers.
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Chiroptical Spectroscopy è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Chiroptical Spectroscopy di Prasad L. Polavarapu in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Ciencias físicas e Química industrial y técnica. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
1
Polarized Light
Interaction between electromagnetic radiation and matter is the basis of a broad research area that is generally categorized as spectroscopy. When the matter under consideration is made up of chiral molecules (see Chapter 2), a special branch of spectroscopy, labeled as chiroptical spectroscopy, becomes pertinent. The words “chiroptical spectroscopy” can be construed to mean the study of chiral systems using optical spectroscopy methods. To understand the principles of chiroptical spectroscopy, it is necessary to gain insight into the properties of electromagnetic radiation, chiral molecules, and the interaction between them. These concepts form the basis for the starting chapters in this book.
1.1 Introduction to Light
Visible light represents electromagnetic radiation that can be detected with the human eye. This statement automatically raises thoughts about “invisible” light. To distinguish between visible and invisible light, we need to consider the wave properties of electromagnetic radiation. A wave (see Figure 1.1a) is associated with periodic oscillatory variation of its height (referred to as amplitude) in time. Two identical points in a wave constitute one cycle. For example, two consecutive crest points, two consecutive trough points, or three consecutive zero points constitute one cycle or one wave. If an observer stands at one point and watches the waves pass by in time, the number of cycles passed in a given second is labeled as frequency (ν). The unit of frequency is cycles/second (or simply s−1), which is designated as hertz (Hz; 1 Hz = s−1). A cosine wave with unit amplitude is represented by the function cos2πν t, where t stands for time in seconds. This cosine wave is shown in Figure 1.1a, where the definition of a cycle is depicted. The alternate representations of frequency of oscillation are wavelength and wavenumber. If a stationary wave is held along the z axis (see Figure 1.1b) and the distance between three consecutive zero points (or between two crests or between two troughs) of that wave is measured, then that distance is labeled as wavelength and represented by symbol λ [expressed as meters/cycle or simply as meters (m)]. This definition of wavelength is shown in Figure 1.1b, where a wave with unit amplitude, represented by the cosine function cos2πz/λ, is depicted. The product of wavelength and frequency of a wave is equal to the speed of that wave c, which is expressed as m·s−1:
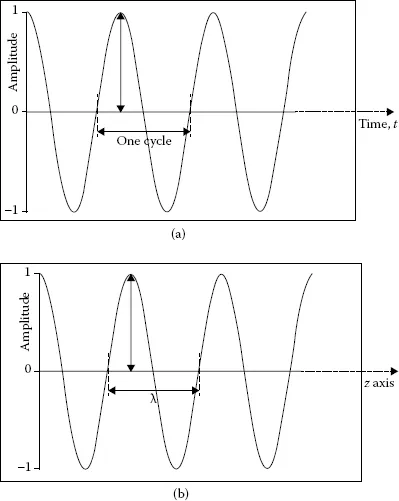
FIGURE 1.1
(a) Depiction of the amplitude and cycle of an oscillating cosine wave, cos 2πνt, with unit amplitude as a function of time. (b) Depiction of the amplitude and wavelength λ of an oscillating cosine wave, cos (2πz/λ), propagating along the z axis with unit amplitude.
(1.1) |
If an electromagnetic wave passes through a medium with refractive index nm, then the speed of electromagnetic wave changes as cm = c/nm. Then, Equation 1.1 modifies as cm = λmν, where λm is the wavelength of the wave in that medium (Levine 2009). For the current purposes, consider the waves propagating in vacuum, where nm = 1. Just as the number of cycles per second is labeled as frequency, the number of waves per unit length is labeled as wavenumber (). Since one wave occupies a length of λ, wavenumber is equal to the reciprocal of wavelength. Therefore, = 1/λ, and its units are expressed in m−1. Thus, an alternate form of Equation 1.1 is as follows:
(1.2) |
The wavelength λ (or equivalently frequency ν or wavenumber ) of a wave determines the properties of that wave. Human eyes respond to the electromagnetic waves of wavelength between ~400 and 800 nm (1 nm = 10−9 m), and this portion of electromagnetic radiation is referred to as the visible light. The different colors that human eyes can recognize (violet, indigo, blue, green, yellow, orange, and red, abbreviated as VIBGYOR) are the components of the visible light. The portions of electromagnetic radiation with wavelengths greater or less than the wavelengths in the visible light range are invisible to the human eye.
From here onward, the word “light” will be used to represent electromagnetic radiation (regardless of whether it is visible or invisible to the human eyes). The speed of light in vacuum is a constant, 2.99792458 × 108 m·s−1. Wavelength and frequency of a light wave are related through Equations 1.1 and 1.2. The different wavelength ranges of electromagnetic radiation constitute different portions of the electromagnetic “spectrum.” Selected portions of electromagnetic spectrum and their names are summarized in Table 1.1.
Wavelength is expressed in nanometers (nm) for the ultraviolet–visible region and micrometers (μm) for the infrared region. Wavenumber is usually expressed in cm−1. For example, a light wave with 200-nm wavelength represents ultraviolet light and corresponds to a wavenumber of 50,000 cm−1 and a frequency of 1.5 × 1015 Hz .
TABLE 1.1
Selected Regions of E...